Bacterial infections in the oral cavity stem from the accumulation of bacteria in the form of biofilms. A biofilm consists of a collection of microbes adhering to any surface in the mouth. Both aerobic and anaerobic bacteria are present in biofilm residing in the oral cavity. An accumulation of anaerobic bacteria, such as Porphyromonas gingivalis, cause the gum to become irritated and can lead to harmful oral diseases like periodontitis. Since anaerobic bacteria are largely present in these biofilms, scientists are developing antimicrobial drugs that selectively target specific functions of anaerobic bacteria and prevent further development of drug resistance. In anaerobic bacteria, part of its metabolic process for energy production is pyruvate oxidation, where pyruvate is converted to Acetyl-CoA. Anaerobic bacteria possess a different enzyme from pyruvate dehydrogenase (the enzyme present in aerobic bacteria for pyruvate oxidation): pyruvate ferredoxin oxidoreductase (PFOR) (see Fig. 1). Kennedy et al’s study focuses on targeting the metabolic process of pyruvate oxidation in anaerobic bacteria.
Kennedy A.J., Bruce A.M., Gineste C., Ballard T.E., Olekhnovich I.N., Macdonald T.L. & Hoffman P.S. (July 2016)
Synthesis and Antimicrobial Evaluation of Amixicile-Based Inhibitors of the Pyruvate-Ferredoxin Oxidoreductases of Anaerobic Bacteria and Epsilonproteobacteria
Antimicrobial Agents and Chemotherapy 60:3980 - 3987
Synthesis and Antimicrobial Evaluation of Amixicile-Based Inhibitors of the Pyruvate-Ferredoxin Oxidoreductases of Anaerobic Bacteria and Epsilonproteobacteria
Antimicrobial Agents and Chemotherapy 60:3980 - 3987
(Translated by Nasita Islam)
Introduction
|
Bacteria can be divided in two categories: aerobic and anaerobic. The majority of aerobic bacteria have an anti-inflammatory response while the majority of anaerobic trigger a pro-inflammatory response. The biofilm in the oral cavity illustrates this distinction.
|

Fig. 1: Comparison of pyruvate metabolism between aerobic and anaerobic bacteria. [Hutcherson et al.] |
|
Along with its cofactor thiamine pyrophosphate (TPP), PFOR converts pyruvate into Acetyl-CoA and CO2, with the generated reducing equivalents are transferred to electron carrier ferredoxin. Specifically, pyruvate and CoASH bind to TPP, which ultimately catalyzes their conversion to Acetyl-CoA. Therefore, a drug that could block pyruvate metabolism would possibly terminate energy production and thus possibly kill the anaerobic bacteria. PFOR has a cofactor in its active site, an adjunct helper molecule, called thiamine pyrophosphate (TPP). Pyruvate and CoA-SH bind to TPP and TPP is what ultimately catalyzes the conversion of Acetyl-CoA. TPP is a crucial cofactor which indirectly catalyzes energy production. Decades ago, studies developed an antimicrobial named nitazoxanide (NTZ) which targeted pyruvate oxidation. Hoffman et al. (2006) used a spectrophotometer to observe how NTZ inactivates PFOR - a decrease in absorbance (with respect to the graph, the line went down) indicated that PFOR has been deprotonated (a proton was taken away). Hoffman et al. suggested that NTZ abstracted a proton from TPP, which inactivated TPP. The inactivated state of TPP thus wouldn’t be able catalyze the conversion of pyruvate to Acetyl-CoA. NTZ was initially advocated as a potential drug for oral diseases. Later studies found that since NTZ is required in large amounts to be effective, it may have harmful side effects. Kennedy et al. attempted to modify the molecular structure of NTZ to find a more effective derivative (a modified molecule). Kennedy at al. found that amixicile was a potentially effective derivative of NTZ because unlike NTZ, amixicile is effective in small amounts. Amixicile is such a candidate drug that is being studied for how it prevents the oxidation of pyruvate. Thus, it is of interest to examine how amixicile binds to PFOR and prevents pyruvate oxidation (see Fig. 2). |
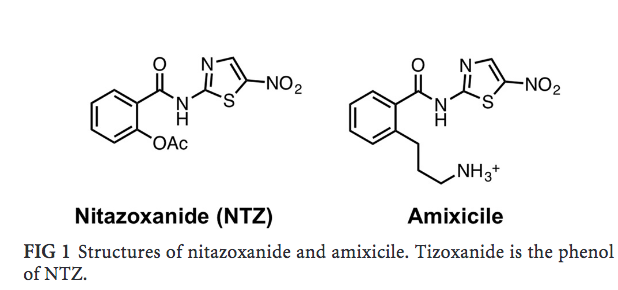
Fig. 2: NTZ and amixicile structure: Note how the main difference between amixicile and NTZ is the extension of a tail in amixicile (propylamine tail). [Kennedy et al.]) |
References
- Hutcherson J.A, Sinclair K.M, Belvin B.R., Gui Q., Hoffman P.S. & Lewis J.S (2017). Amixicile, a novel strategy for targeting oral anaerobic pathogens. Scientific Reports 7:10474.
- Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. (2007). Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori and selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob Agents Chemother 51: 868 – 876.