Enzyme assays and MIC (minimum inhibitory concentration) tests were both pharmacologically focused experiments. Both were implemented to observe the observe the rate of inhibition brought by each amixicile analogue (which was virtually and chemically synthesized via SAR and Suzuki coupling, respectively). The enzyme assay measured the percentage of inhibition of PFOR specifically while the MIC tests measured the percentage of inhibition of the bacteria overall. Procedure: Enzyme Assay
The PFOR of each bacterium (H. pylori, C. difficile., & C. jejuni) was amplified and isolated from E. coli. To prepare the PFOR for assay, potassium phosphate, sodium pyruvate, and benzyl viologen were added under anaerobic conditions - this procedure would prepare the PFOR for quantitative analysis so that inhibition can be measured via spectrophotometer. PFOR activity was initiated with or without an inhibitor (i.e. NTZ, amixicile, and the amixicile analogues) along with the addition of redox-active BV dye, which would act as an indicator of inhibition for the spectrophotometer, specifically any reduction activity would be highlighted by the BV dye.
Procedure: MIC Test
H. pylori and C. difficile were prepared differently for the MIC test, specifically via microdilution and agar dilution respectively.
Subprocedure: Agar dilution (C. difficile)
The bacteria were grown in a chopped-meat medium overnight, then transferred to fresh chopped-meat medium and let sit for 5 hours. The inhibitors (i.e. NTZ, amixicile, and the amixicile analogues) were separately diluted in agar media. Ten microliters of the bacteria-media mixture were then dispensed on each agar-inhibitor plate. The plates were incubated for 18 hours anaerobically and then observed with the naked eye for signs of growth.
Subprocedure: Microdilution (H. pylori & C. jejuni)
The bacteria were grown in medium consisting of either brucella broth and brain heart. The cultures were diluted and placed in a 96-well microplate. The inhibitors (i.e. NTZ, amixicile, and the amixicile analogues) were serially diluted in the microplate. The microplate was then incubated and shaked at 37C aerobically. The turbidity in each well was then measured. Since turbidity usually indicates bacterial growth, the MIC value was calculated according to the concentration of the inhibitor in the well that had no detectable turbidity.
|
Result
In figure 7, the R values (NOT the R-group itself) represent the variables destined to be replaced with different moieties (legend circled in green). The amixicile analogues (R groups of analogues circled in purple) were chemically synthesized via <A href=“expt2.html" target="_blank">Suzuki coupling</A> . The cLogP value (red arrow) determines the hydrophilicity of the specific R group - the higher the LogP value, the lower the hydrophilicity and therefore absorption. The PFOR inhibition value (blue arrow) attributes a percentage of inhibition of PFOR activity with each amixicile analogue (based on the spectrophotometer readings). Variations of the R group of the amixicile analogues were tested against the PFOR of H. pylori, C. difficile, and C. jejuni. The PFOR inhibition value attributes a percentage of inhibition of PFOR activity with each amixicile analogue (based on the spectrophotometer readings). It appears that the highest percentage of PFOR inhibition is 97%, is attributed to compound 4.10 (an analogue of amixicile). The MIC value is the minimum concentration of the inhibitor compound (in micrograms per microliters) where no turbidity was observed, presuming bacterium death. Note how for each bacteria the MIC value varies greatly - the MIC value for each compound is irregular across the bacteria, which may suggest that amixicile inhibits each anaerobic bacteria at slightly different concentrations. C. difficile overall has the lowest MIC values for all the compounds, indicating that small amounts of amixicile inhibit the overall biological activity of amixicile. Alternatively, C. jejuni overall has relatively high MIC values, indicating that slightly higher amounts of amixicile inhibit the overall biological activity of amixicile. Unlike the patterns observed in figure 6 with compounds 4.1 to 4.5, there is no discernable pattern in this figure among the PFOR inhibition percentages and MIC values. Nevertheless, amixicile analogue 4.8 has well-rounded PFOR inhibition percentage and relatively low MIC values: 93% for PFOR inhibition percentage and MIC values of 0.125, 0.125, and 4 ?m/mL for H. pylori, C. difficile, and C. jejuni respectively. Note how the NTZ PFOR inhibition percentage is smaller compared to the other compounds but its MIC value varies compared to the other compounds. To elaborate, the NTZ MIC value of C. difficile is relatively greater than that of amixicile and its analogues. on the other hand, the NTZ MIC value of C. jejuni is relatively low compared to that of amixicile and and its analogues. The NTZ MIC value of H. pylori is relatively similar to half of that of amixicile and its analogues and higher than that of the other half. |
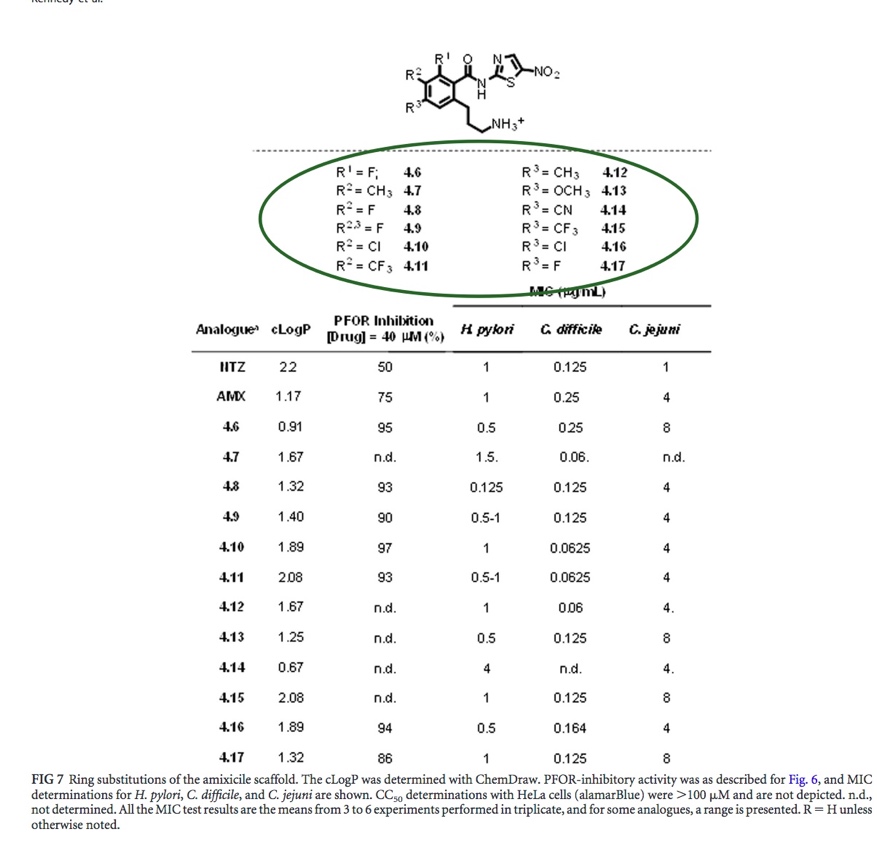
Fig. 7: Enzyme and MIC activity results [Kennedy et al.].
|