Part I - Defining a Radiopharmaceutical
- What is a radiopharmaceutical?
- Pharmaceutical is an element or compound that can be utilized by the body
- Is has specific biokinetics and will follow a specific biodistribution (physiology) when absorbed by the body
- Tagging a compound or chelating it to a radioactive element makes it a (radio + pharmaceutical =) radiopharmaceutical
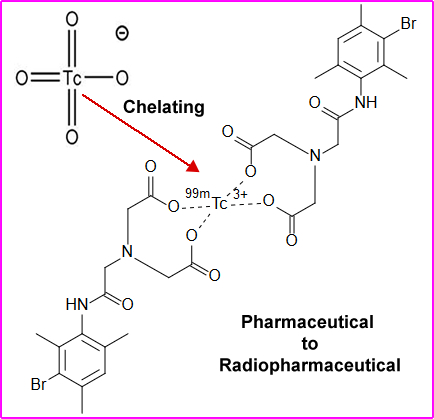
- When compounding (making) a radiopharmaceutical it must not alter the pharmaceutical biodistribution or physiological properties of the agent. As in the above example 99mTc is bound to DISIDA. For more information on 99mTcDISIDA follow the link
- This radioactive tracer evaluates hepatobiliary function
Part II - Types of Radiopharmaceutical Biodistribution
- Simple Diffusion/Passive Transport
- High concentration moving to areas of low concentrations
- Small molecules or atoms that can move across cell membranes without exerting any energy
- Pertechnetate scan is a combination of simple diffusion and active transport. It depends on what system with the body we are discussing
- Its molecular weight is 161.9 g/mol
- Active Transport
- Movement of a molecule of atom across a cell membrane
- The process involves the expenditure of energy
- Usually results in higher concentration of the involved element(s) within the targeted cell/organ
- Examples:
- 201Tl in myocardial tissue
- 123I in thyroid tissue
- Compartmentalization
- Element of compound that becomes trapped in a "compartment" within the body
- Examples
- 99mTc labeled RBCs for analysis of myocardial contraction/GI bleeding
- 111In DTPA in cerebral spinal fluid (Cisternogram)
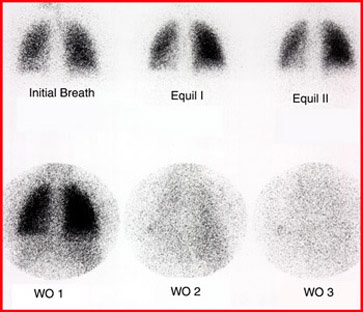
- Lung ventilation with either: 99mTc DTPA Aerosol or 133Xe
- Capillary/Arteriole Blockage
- Trapping of particles (microembolization) in the arteriole structure of the vascular cavity
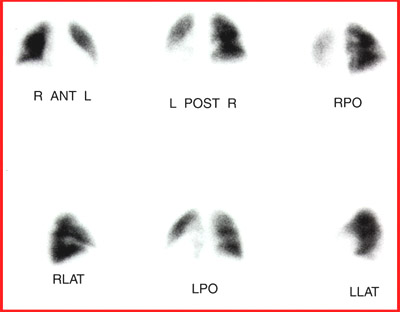
- Example - 99m TcMAA particles trapped in the arterioles of the lung
- So when we add MAA and Xe (or DTPA) together looking for pulmonary emboli (PE). PE is identified when there is lack of uptake with MAA (vascular) and uptake seen in ventilation with DTPA or Xe. Important concept - think physiology ..
- Cell Sequestration
- Splenic sequestration
- Example - Damaged RBCs are then labeled with 99mTc and the spleen is then imagined - splenic sequestration
- Phagocytosis
- Cells that trap or "grab" particles
- Example - phagocytosis of 99mTcSulfur Colloid particles by the Reticular Endothelial Cells (RES/REC). In the liver, Kupffer cell trap these particles, but REC can also be found in the spleen and bone marrow
- Chemisorption
- Binding of a chemical/substance to a solid surface forming a chemical bond with the molecule that comes in contact with that surface
- Secondary requirement - Requires blood flow to that area for this process to occur
- Example
- 99m TcMDP binds onto the hydroxyapatite crystal also referred to as the bony matrix
- The better the blood supply the greater the bone uptake
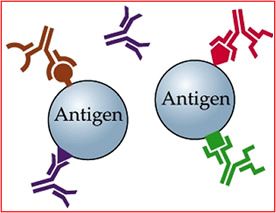
- Antibody/Antigen reaction
- Lock and key relationship
- The antibody's surface has a specific code on its surface that allows it to bond a specific antigen
- Example
- Monoclonal antibodies bind to tumor surface
- ProstaScint (In111 capromab pendetide) binds to tumors specific to prostate cancer
- Receptor/Binding
- Bonding of compound to a specific receptor site - peptides bind the surface of disease
- Similar to the antibody/antigen reaction, however, the radiopharmaceutical is not a monoclonal antibody
- Example
- 111In labeled Octreotide binds to the surface of neuroendocrine tumor
- Octreotide is a somatostatin analog or derivative
- Synthetic peptide usually have fewer amino acids
- Example of 131I vs 111In octreotide detection from Hurhle cell carcinoma at the metastatic level. Octreotide is a lot more sensitive in finding disease
Part III - Matching the "Ideal Radiopharmaceutical" with a Gamma Camera
- Matching available instrumentation to the specifics of a radiopharmaceutical
- Crystal thickness (3/8")
- Crystal thickness relates to the efficiency of counting
- Made for a 140 keV gamma
- For a higher energy gamma, crystal thickness should be increased, however, replace the crystal is virtually impossible
- Thickness of the crystal also relates to the resolution of the system - ideal for 99m Tc
- Pure gamma emitter
- Reduces radiation burden to the patient
- Mixed emitters that contain beta or alpha radiation will increase the radiation burden
- Energy gamma
- Crystal thickness of 3/8" is designed for 140 keV gamma
- Energy gamma less than 100 keV will have greater attenuation in the body and fewer will reach the crystal
- Energy gamma higher that 200 keV reduces body attenuation, however, decreases the chance of being detected by the crystal (would need to increase the thickness of the crystal)
- Count density
- Greater the dose translates to more counts
- More counts means increased count density resulting in better resolution
- Half-Live
- Shorter half-life allows for higher dose
- Reduced radiation burden
- Allows for greater amount of activity to be injected = greater counts
- Inexpensive and availability
- 99mTc is readily available and inexpressive
- Cyclotron produced radiopharmaceuticals are not as readily available and are usually more expensive
- How does 99mTc fit into this equation? Evaluate the following
- Crystal thickness
- Pure vs. mixed emitter
- Energy gamma
- Count density
- Resolution
- Radiation burden
- Half-Life
- Expense and availability
Return to the beginning of the document
Return to the Table of Contents
End of this Week's Lectures
5/20