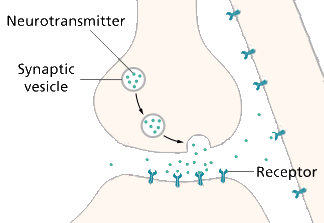
Recent technological advances have made possible more accurate measurements of glutamate in and near cells [
10Tsien RY (2005) Building and breeding molecules to spy on cells and tumors. FEBS Lett 927–932.,
11Okumoto S, Looger LL, Micheva KD, Reimer RJ, Smith SJ, Frommer WB (2005) Detection of glutamate release from neurons by genetically
encoded surface-displayed FRET nanosensors. Proc Natl Acad Sci USA 102:8740–8745.]. The method [
12Deuschle K, Okumoto S, Fehr M, Looger LL, Kozhukh L, Frommer WB (2005). Construction and optimization of a family of genetically encoded metabolite sensors by semirational protein engineering. Prot Sci, 14:2304–2314.], illustrated in
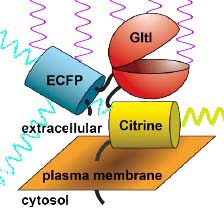
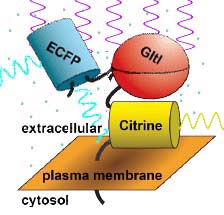
Fig. 2 relies on two proteins that are intrinsically fluorescent, fused to a third protein that controls the proximity of the two fluorescent proteins, depending on the presence of glutamate. One protein,
Enhanced Cyan Fluorescent Protein (ECFP) fluoresces blue-green light when exposed to violet light. The second,
Citrine, a type of Yellow Fluorescent Protein (YFP) fluoresces yellow-green light when exposed to blue-green light. The two proteins were fused to a third --
glutamate periplasmic binding protein (GltI), which changes its shape when it binds glutamate. The concentration of glutamate can therefore be detected by the amount of yellow fluorescence emitted in response to violet light: the more glutamate is present, the more is bound to
GltIglutamate-binding protein, and the resulting changein protein conformation pushes
ECFPenhanced cyan fluorescent protein away from
Citrinea type of yellow fluorescent protein. This leads to less aborbance of blue-green light by
Citrinea type of yellow fluorescent protein and less yellow-green fluorescence.
Unfortunately, existing fluorescent glutamate detectors show only a small difference in yellow-green fluorescence in response to glutamate. The authors sought alterations in the structure of the original detector that showed a greater difference and then used this modified detector to monitor glutamate levels near neurons in real time.