In Graph 2, the x-axis shows the varying Fructose-6-P concentrations in mM, and the y-axis shows the activity of PfkB in U/mg (units per mg). Two slopes are present, the one with +HPr is with presence and the one with -HPr is with absence. The slope with +HPr shows a quick activation of PfkB at low Fructose-6-P concentration, showing a higher affinity for PfkB for Fructose-6-P, hitting the Vmax of about 40 U/mg. The slope with -HPr has a Vmax of around 41 U/mg, which was close to the Vmax of +HPr. The Khalf seems to be around .08 for +HPr and .49 for -HPr. The ratio for -HPr / +HPr is .49 / .08, which is around 8, leading to the observation that the presence of +HPr caused the slope to decrease 6-fold, which is seen in the graph.
Rodionova IA, Zhang Z, Mehla J, Goodacre N, Babu M, Emili A, Uetz P, Saier MH Jr. (2017)
The phosphocarrier protein HPr of the bacterial phosphotransferase system globally
regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli
J Biol Chem. 2017 Aug 25;292(34):14250-14257
The phosphocarrier protein HPr of the bacterial phosphotransferase system globally
regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli
J Biol Chem. 2017 Aug 25;292(34):14250-14257
(Translated by Shravani Wadwekar)
Experiment 2: Allosteric activation of PfkB by HPr
|
HPr and PfkB activity was measured by performing HPr dependent allosteric regulation (when a ligand - a molecule or ion - binds to a site, other than the active site, to regulate activity) through a coupled assay involving coupled assay involving Fructose-6-P and LDH. 2.2 uM of HPr or HPr-P were added to an assay mixture. In this experiment, HPr had an effect. HPr was then incubated (set in a favorable environment that stimulates growth or development) for 40 minutes at 30 degrees Celsius. In a separate reaction mixture, 24 ng of PfkB was added to 100 uL of a reaction mixture containing several concentrations of substances including 0-3 mM Fructose-6-P, 0.3mM NADH, 1.2 units PK, 1.2 mM ATP, and 1.2 units LDH. 2.2 uM HPr from the first assay mixture were then added to the PfkB reaction mixture. The PEP was converted to pyruvate by PK, dependent on the conversion of the ATP to ADP, as the ADP was used by PK in the conversion of PEP to pyruvate; LDH then reduced the pyruvate to lactate. During the second reaction, NADH was oxidized to NAD+; the NADH to NAD+ oxidation concentration in the presence and absence of HPr was measured to give a graph of steady-state kinetics (when all state variables are constant; everything but the varying concentrations of Fru-6P). Steady state kinetics include the Khalf, Vmax, and the Hill Coefficient, shown in Table 2.
|
|
|
|
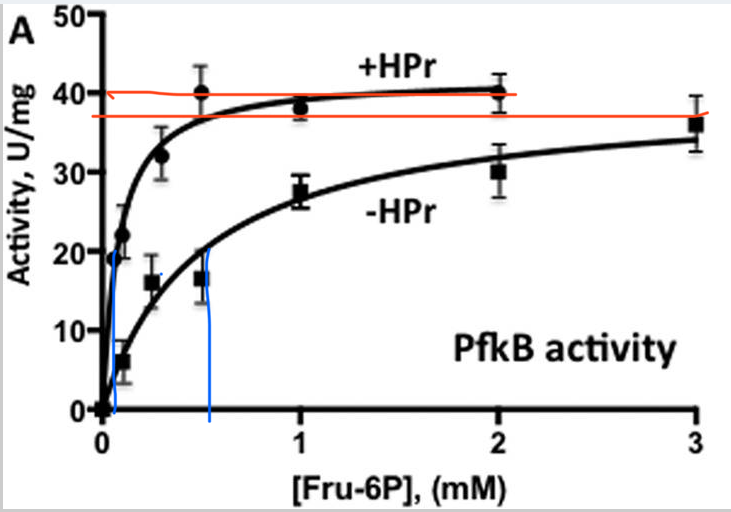
Graph 2. Allosteric activation of PfkB by HPr. Graph shows PfkB activity dependent on varying Fru-6-P concentration and absence or presence of HPr. The presence of HPr is identified by +HPr and the absence of HPr is identified by -HPr. The graph exhibits the Vmax, which is shown by orange lines, and the Khalf, which is shown by blue lines.
Adapted from "The phosphocarrier protein HPr of the bacterial phosphotransferase system globally regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli." by Rodionova IA, Zhang Z, Mehla J, Goodacre N, Babu M, Emili A, Uetz P, Saier MH Jr., 2017, J Biol Chem, Page 14253. |
|
|
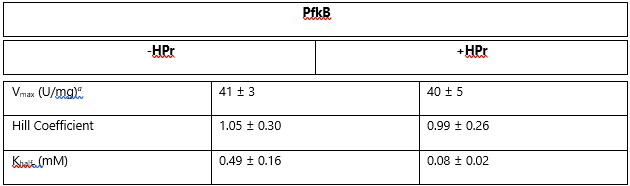
Table 2. Steady State Kinetics of PfkB in the absence (-HPr) and presence (+HPr) of HPr. Table shows the Vmax, Hill Coefficient, and Khalf of the activity. They are visually represented in Graph 2.
Adapted from "The phosphocarrier protein HPr of the bacterial phosphotransferase system globally regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli." by Rodionova IA, Zhang Z, Mehla J, Goodacre N, Babu M, Emili A, Uetz P, Saier MH Jr., 2017, J Biol Chem, Page 14252. |
|
|
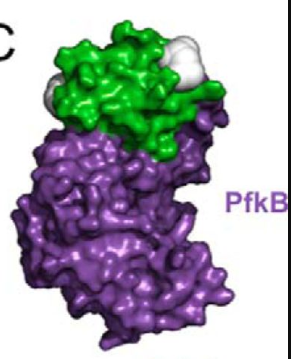
Figure 2. Complex of HPr and PfkB. Figure shows the complex of HPr and PfkB. HPr is represented by green and PfkB is represented by purple.
Adapted from "The phosphocarrier protein HPr of the bacterial phosphotransferase system globally regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli." by Rodionova IA, Zhang Z, Mehla J, Goodacre N, Babu M, Emili A, Uetz P, Saier MH Jr., 2017, J Biol Chem, Page 14254. |
|
|