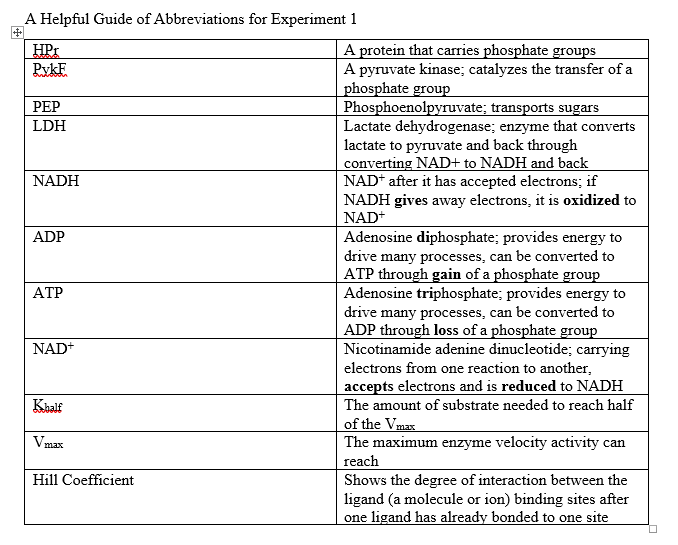
In Graph 1 the x-axis shows the varying PEP concentrations in mM, and the y-axis shows the activity of PykF in U/mg (units per mg). Two slopes are present, the one with +HPr is with presence and the one with -HPr is with absence. The slope with +HPr shows a quick activation of PykF at low PEP concentration, showing a higher affinity of PykF for PEP, hitting the Vmax of about 117 U/mg. The slope with -HPr has a sigmoidal shape (S shape) showing a Vmax of around 120 U/mg, which was close to the Vmax of +HPr. The Khalf seems to be around .36 mM for +HPr and 3.5 mM for -HPr. The ratio for -HPr / +HPr is 3.5 /.36, which is around 10, leading to the observation that the presence of HPr caused the slope to decrease 10-fold, which is seen in the graph.
Rodionova IA, Zhang Z, Mehla J, Goodacre N, Babu M, Emili A, Uetz P, Saier MH Jr. (2017)
The phosphocarrier protein HPr of the bacterial phosphotransferase system globally
regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli
J Biol Chem. 2017 Aug 25;292(34):14250-14257
The phosphocarrier protein HPr of the bacterial phosphotransferase system globally
regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli
J Biol Chem. 2017 Aug 25;292(34):14250-14257
(Translated by Shravani Wadwekar)
Experiment 1: Allosteric activation of PykF by HPr
|
HPr and PykF activity was measured by performing HPr-dependent allosteric regulation (when a ligand - a molecule or ion - binds to a site, other than the active site, to regulate activity) through a coupled assay involving PEP and LDH. 2 uM of HPr or HPr-P were added to an assay mixture. In this experiment, HPr had an effect. HPr was then incubated (set in a favorable environment that stimulates growth or development) for 40 minutes at 30 degrees Celsius. In a separate reaction mixture, 15 ng of PykF were added to 100 uL of a reaction mixture containing several concentrations of substances including 0-8 nM of PEP, 0.3 mM of NADH, 1.5 mM of ADP and 1.2 units of LDH. 2 uM HPr or HPr-P from the first assay mixture were then added into the PykF reaction mixture. The PEP was converted to pyruvate by PykF in an ADP-dependent enzymatic reaction. The pyruvate was used as the substrate and converted to lactate by LDH in the second enzymatic reaction in the presence (+HPr) or absence (-HPr) of HPr. During the second enzymatic reaction, the NADH was oxidized to NAD+; the NADH to NAD+ oxidation concentration in the presence and absence of HPr was measured to give a graph of the steady-state kinetics (when all state variables are constant; everything but the varying concentrations of PEP). Steady state kinetics include the Khalf, Vmax, and the Hill Coefficient, shown in Table 1.
|
|
|
|
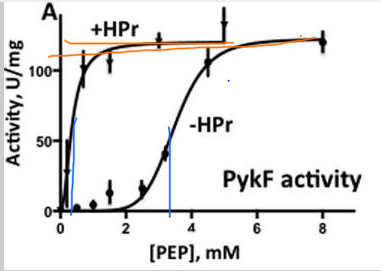
Graph 1. Allosteric activation of PykF by HPr. Graph shows PykF activity dependent on varying PEP concentration and absence or presence of HPr. The presence of HPr is identified by +HPr and the absence of HPr is identified by -HPr. The graph exhibits the Vmax, which is shown by orange lines, and the Khalf, which is shown by blue lines.
Adapted from "The phosphocarrier protein HPr of the bacterial phosphotransferase system globally regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli." by Rodionova IA, Zhang Z, Mehla J, Goodacre N, Babu M, Emili A, Uetz P, Saier MH Jr., 2017, J Biol Chem, Page 14252. |
|
|
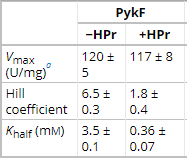
Table 1. Steady State Kinetics of PykF in the absence (-HPr) and presence (+HPr) of HPr. Table shows the Vmax, Hill Coefficient, and Khalf of the activity. They are visually represented in Graph 1.
Adapted from "The phosphocarrier protein HPr of the bacterial phosphotransferase system globally regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli." by Rodionova IA, Zhang Z, Mehla J, Goodacre N, Babu M, Emili A, Uetz P, Saier MH Jr., 2017, J Biol Chem, Page 14252. |
|
|
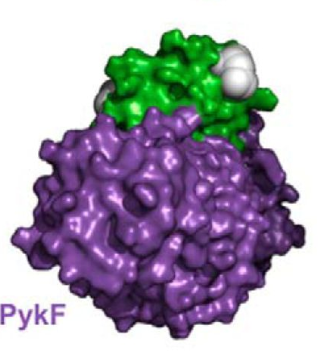
Figure 1. Complex of HPr and PykF. Figure shows the complex of HPr and PykF. HPr is represented by green and PykF is represented by purple.
Adapted from "The phosphocarrier protein HPr of the bacterial phosphotransferase system globally regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli." by Rodionova IA, Zhang Z, Mehla J, Goodacre N, Babu M, Emili A, Uetz P, Saier MH Jr., 2017, J Biol Chem, Page 14254. |
|
|