Figure 5 shows the gel analysis comparing the exonuclease activity. The DNA is marked with a dye so it can be seen being passed through the gel. The image is labeled with a + and - end. DNA is negatively charged so for the gel to start working an electric current is ran through the gel allowing the DNA fragments to form. The result is a series of 'bands', with each band containing DNA molecules of a particular size. The bands towards the minus end contain the smallest fragments of DNA. While, the bands closet to the plus end contain the largest DNA fragments. The bands form over time and you will notice a light grey area forming at the ends of the DNA strand. The end of the DNA strand is marked with a red arrow and this pointing at the site at which the nucleotides are being cleaved. The band starts with a 24mer sequence and as it runs towards the negative end the exonuclease is continuously cleaving the ends, taking the nucleotide out. This goes from a 24mer sequence to 23, 22, 21, and until the exonuclease has cleaved all the nucleotides out. Why are all the nucleotides being cleaved you might be wondering. This is occurring because when the duplex was mixed with the MgCl. This compound causes the exonuclease to activate and begin cleaving.
Joy Y. Feng, Allison A. Johnson, Kenneth A. Johnson and Karen S. Anderson(2001)
Insights into the Molecular Mechanism of Mitochondrial Toxicity by
AIDS Drugs*
The Journal of Biological Chemistry Vol.276,No.26, Issue of June, pp.23832-23837
Insights into the Molecular Mechanism of Mitochondrial Toxicity by AIDS Drugs*
The Journal of Biological Chemistry Vol.276,No.26, Issue of June, pp.23832-23837
(Translated by Owais A. Shahzada)
Experiment:Locating exonuclease activity
|
To check for the exonuclease removal of the nucleotides of (+) and (-)3TC-MP. [explain how it is a monophosphate]. The 24mer sequence is analyzed through gel-electrophoresis and what was discovered was the rate at which the nucleotides were being cleaved by the exonuclease from 3'-5' ends. For this gel analysis to work the DNA was synthesized and quenched by the KinTek instrument. The process began with an accessory subunit, catalytic subunit, and a 24/45mer DNA duplex in one syringe. In another syringe contained a MgCl2. Both the syringes were initiated and mixed. A chemical known as EDTA is then added to stop the mixture of MgCl2 and the DNA duplex at various time points. Once the solution pours into a test tube the steps for gel analysis can begin to check for exonuclease activity.
|
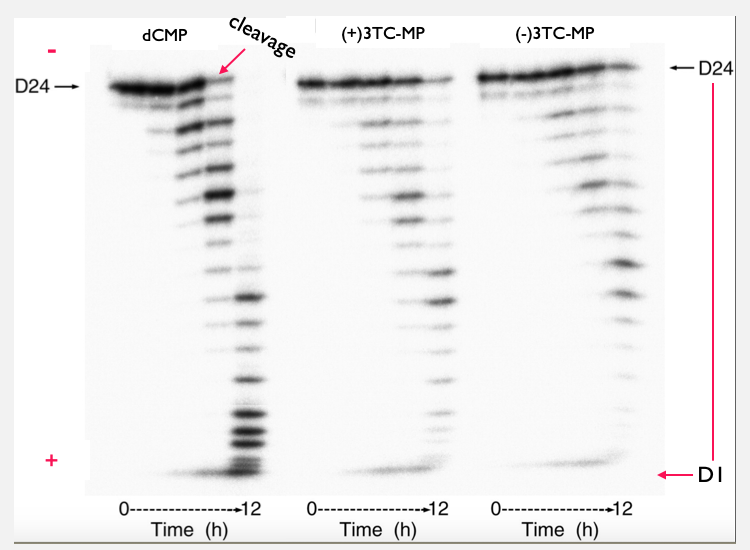
Fig. 6: Gel-analysis comparison of the exonuclease activity. . A gel analysis of three different 24mer DNA duplex were compared. The figure contains the dCTP wild-type and the two configurations of 3TC. The gel analysis illustrates if the mitochondrial DNA polymerase is making corrections by cleaving off the incorrect nucleotide and the rate at which the excisionion occurs over a period of 12 hours.
|
|
|