Joy Y. Feng, Allison A. Johnson, Kenneth A. Johnson and Karen S. Anderson(2001)
Insights into the Molecular Mechanism of Mitochondrial Toxicity by
AIDS Drugs*
The Journal of Biological Chemistry Vol.276,No.26, Issue of June, pp.23832-23837
Insights into the Molecular Mechanism of Mitochondrial Toxicity by AIDS Drugs*
The Journal of Biological Chemistry Vol.276,No.26, Issue of June, pp.23832-23837
(Translated by Owais A. Shahzada)
Abstract
|
|
|
|
HIV, a viral infection which occurs through the contact of bodily fluids, is a virus that takes over the hosts cells and integrates its DNA into the human chromosome. The replication machine that HIV uses is known as reverse transcriptase (RT). This enzyme (RT) copies the viral RNA genome into DNA before integration. Over time as the virus spreads throughout the patient, their immune defense system fails and weakens the body overtime.
Patients are prescribed to take antiviral drugs to slow the replication process of viral RNA into DNA. Some of the drugs consumed by the patients have shown levels of toxicity which inhibit their usefulness. Toxicity of the drugs may be affecting the human mitochondrial DNA polymerase (Pol gamma). The function of the polymerase is to create new strands (synthesize) of mitochondrial DNA through making an exact copy of a template strand. The polymerase also has a proofreading mechanism (an exonuclease) to locate and repair any mistake made within in the DNA strand. The antiviral drugs stop the replication of viral DNA; however, the drugs are also stopping DNA polymerase from creating new mitochondrial DNA. This is shown to be causing dysfunctions within the patients. During the 1990's the popular drug lamivudine was approved by the FDA for clinical use. The drugs given to the patients are nucleoside analogs of the wild-typeNatural occuring nucleotide building block dCTP (Deoxycytidine triphosphate) composed of a base, a sugar and a phosphate. The analogs are modified dCTP, where the sugar portion contains a sulfur instead of an oxygen. The analogs that are compared back to the dCTP are enantiomerspair of molecules that are mirror images of each other (+) and (-)3TC (Fig.2). A nucleoside analog's function is to inhibit the production of the viral DNA by acting as a mimic of the wild-typeNatural occuring nucleotide nucleotide. Experiments have been run to compare the two analogs to wild-type nucleotide to look at how effective the analogs are when incorporated into the mitochondrial DNA. The experiments look at the efficiency of the nucleotide being incorporated into DNA by mitochondrial DNA polymerase. Both (+) and (-)3TC are not a good substrate when incorporating into the DNA by mitochondrial DNA polymerase. Poor substrates like the analogs are presumed to therefore be less toxic to the patient's mitochondrial DNA. (-)3TC may be a better analog compared to its counterpart because it does not get incorporated into the DNA strand as quickly, making it potentially less toxic. Finally, the mitochondrial DNA polymerase proofreading mechanism is able to fix any incorrect matches in the mitochondrial DNA sequence, and also can remove these analogs from the DNA. The study will exhibit how to the drugs play a major role in the function of the DNA polymerase which will lead to better drug designs in the future. |
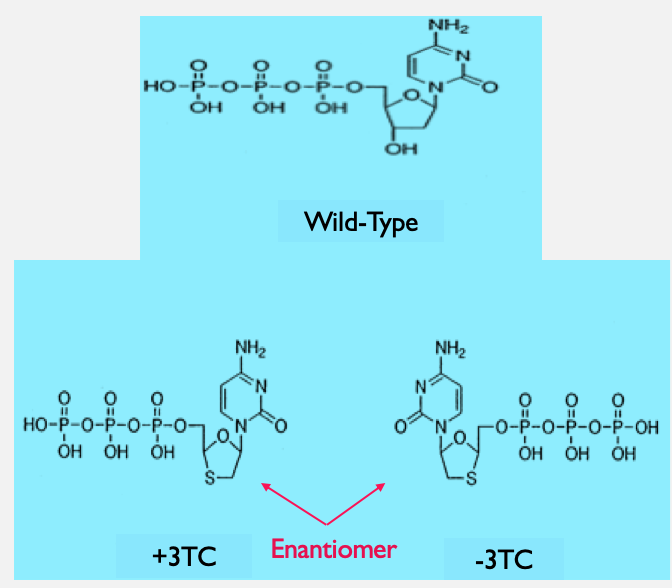
Fig. 2: Deoxycytidine and Nucleoside analogs: The image contains the wild-type(dCTP) and the two-nucleoside that are mirror images of each other known as enantiomers (+) and (-) 3TC. The 3TC enantiomers is the drug lamivudine that is given to patients. |