To understand the mechanism of ZBTB7A regulation, the authors would like to know first whether ZBTB7A is regulated by KLF1 and whether this regulation is achieved by direct binding of KLF1 to the promoter of ZBTB7A gene. The focus of this summary is to explain the Chromatin Immunoprecipitation – Sequencing (ChIP-Seq) experiment that was used to show whether regulation of ZBTB7A by KLF1 is achieved by direct binding of KLF1 to the promoter of ZBTB7A gene.
Laura J. Norton, Alister P.W. Funnell (2017)
KLF1 directly activates expression of the novel fetal globin repressor ZBTB7A/LRF in erythroid cells
Blood Advances 1:685-692
KLF1 directly activates expression of the novel fetal globin repressor ZBTB7A/LRF in erythroid cells
Blood Advances 1:685-692
(Translated by Wassal Alhammad)
Introduction
|
Human hemoglobin is a tetrameric protein that is responsible for transporting oxygen from the lung to the various tissues and organs of human body. Part of this hemoglobin is expressed from a cassette of genes called human β- locus. This locus contains three different types of genes that makes three different types of hemoglobin: Embryonic, Fetal (HbF), and Adult hemoglobin (HbA), shown in Figure 3a. There are two devastating diseases that affect the adult version of hemoglobin, Sickle cell anemia (Fig. 3b)[3] and β-thalassemia. These two diseases will result in decreased supply of oxygen to the tissues. One suggested strategy to treat this disease is to reactivate the fetal hemoglobin (HbF) which does not have the disease that affect adult hemoglobin. Previous studies showed that in adult cells BCL11A is responsible for repressing the production of HbF. This repressor protein is regulated by Kruppel-like transcription factor 1 (KLF1). KLF1 is a master regulator of many cellular mechanism inside erythroid cells which make red blood cells that carry hemoglobin. ZBTB7A is another repressor protein that works similar to BCL11A in repressing the production of HbF. Unlike BCL11A, not much is known about the regulation of ZBTB7A expression. Understanding such mechanism of regulation is important as an alternative pathway for treatment strategies of Sickle cell anemia and β-thalassemia.
|

Fig. 3a: Hemoglobin Switching: as seen in this figure, x-axis (Gestational Age) represents the age of empyo, starting from 0, age of conception. the y-axis indicates how much hemoglobin foun in each types of hemeglobin. For example, Embryonic hemeglobin, blue line has 60% of hemeglobin in first mounth of pregnancy, and drops to 0%, shut down in the 3rd month. Fetal hemeglobin (HbF) (red line) also activates in 1st month and the amount of hemeglobin increases in 4 and 5 month of pregnancy, it start to deactivates before getting birth. while Adult hemoglobin (HbA) (gray line), activates around the 4 month and the switching start in the cross over region, when the Fetel start to deactivates and the adult start to activates/ increases. Modified from semanticscholar website. |
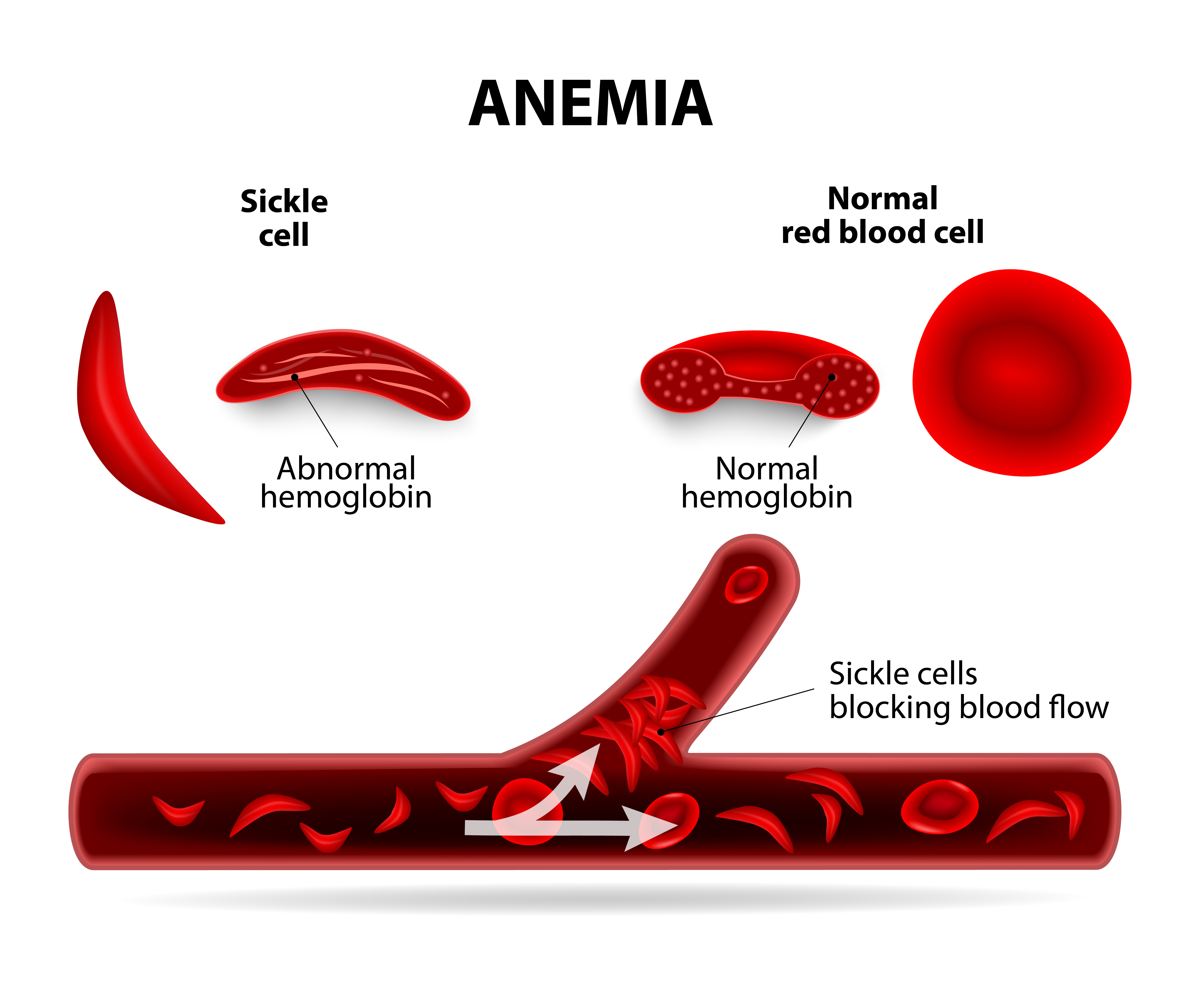
Fig. 3b: comparison of a normal red-blood and sickle-cell cells: The normal cells, which has a normal hemeglobin protein, transpost oxygen from to the cells perfectly (right), but the sickle-cell, which has an abnormal hemeglobin protein, (left) block the blood flow, resulting in blood clots (bottom) .From CIRMWEB (2015). |
|
Any cells contain nucleus and inside it there are chromosomes. These chromosomes are made of DNA and various types of proteins such as histones and other DNA bound-proteins. The composite of DNA and proteins is called Chromatin. A class of DNA binding proteins is called transcription factors. These proteins may promote transcription of some genes or prevent a gene from being expressed. To find whether a protein is actually bind directly to DNA and therefore, promote or suppress gene expression, we use a ChIP-Seq experiment. ChIP-Seq is an abbreviation for Chromatin Immunoprecipitation followed by DNA sequencing [2]. The purpose of Chip-Seq experiment is to locate where in the genome a specific protein bind. In our case we aim to used ChIP-Seq to figure out where in the ZBTB7A gene does KLF1 binds. |
References
- Laura J. Norton, Alister P.W. Funnell (2017). KLF1 directly activates expression of the novel fetal globin repressor ZBTB7A/LRF in erythroid cells. Blood Advances 1:685-692.
- Schmidt D, Wilson MD, Spyrou C, Brown GD, Hadfield J, Odom DT. ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. Methods. 2009;48(3):240-248.
- One-Time, Lasting Treatment for Sickle Cell Disease May be on Horizon, According to New CIRM-Funded Study. (2015) CirmWeb.