
Neuroendocrine Tumors - Diagnostic and Treatment
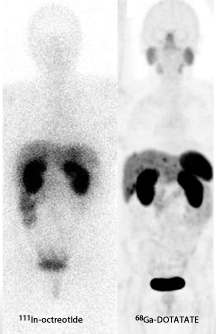
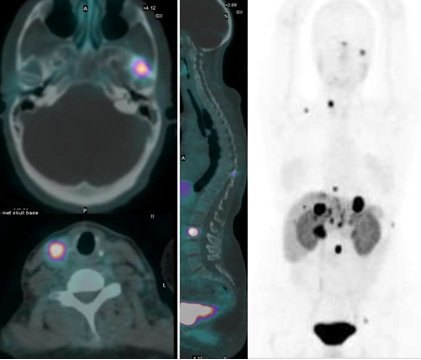
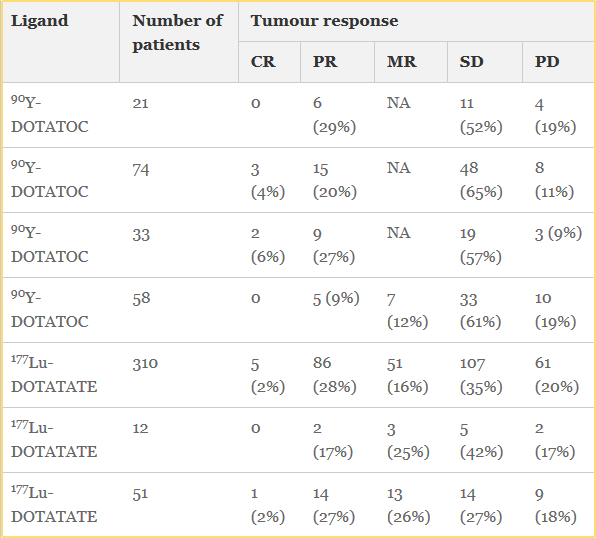
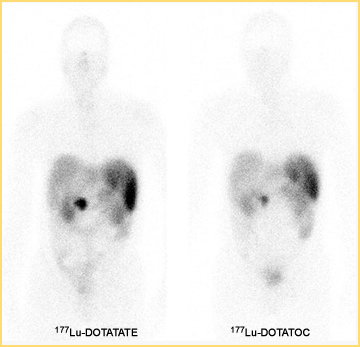
While we are familiar with the use of MIBG and Octreotide PET imaging and therapy has now entered the nuclear arena to evaluate and treat neuroendocrine tumors (NET). We will first explore the imaging of, with PET and then continue with therapy and treatment.




Components of the kit:
Vial 1 (reaction vial with lyophilized powder) contains: 40 mcg dotatate, 5 mcg 1,10-phenanthroline;
6 mcg gentisic acid; 20 mg mannitol.
Vial 2 (buffer vial) contains: 60 mg formic acid; 56.5 mg sodium hydroxide and water for injection.
Accessory cartridge contains: 660 mg porous silica. The accessory cartridge reduces the amount
of Ge 68 potentially present in generator eluate.
Prepare Ga 68 dotatate for intravenous injection according to the following aseptic procedure (see above diagram):
a. Use suitable shielding to reduce radiation exposure.
b. Wear waterproof gloves.
c. Set the temperature of the shielded dry bath to 203 °F (95 °C), and wait for the temperature to reach the set point and stabilize.
d. Prepare a syringe containing 5 mL of 0.1 N sterile HCl, to be used for elution of the GalliaPharm
generator. Use 0.1N sterile HCl supplied by the generator manufacturer. Test periodically (weekly) the Ga 68 chloride eluate for Ge 68 breakthrough by suitable method. Ge 68 breakthrough and
other gamma emitting radionuclides should be ≤ 0.001%. The Ga 68 chloride is sterile as eluted from the GalliaPharm generator.
e. Remove the cap from Vial 1 (reaction vial), swab the top of the vial with alcohol to disinfect the surface, and allow the stopper to dry.
f. Pierce the Vial 1 septum with a sterile needle connected to a 0.22 micron sterile vented filter (not supplied) to maintain atmospheric pressure within the vial during the reconstitution process.
g. Remove the cap from the Vial 2 (buffer vial), swab the top of the vial with alcohol to disinfect the surface, and allow the stopper to dry.
h. Using a 1 mL sterile syringe, withdraw the required volume of the reaction buffer from Vial 2.
Calculate the volume (in mL) by multiplying the volume of HCl used for the elution of the generator in mL by its molarity:
Reaction buffer volume in mL = HCl volume in mL x HCl molarity (for the GalliaPharm generator eluate, 5 mL x 0.1 N = 0.5 mL of reaction buffer).
i. Connect the top of the cartridge to the male luer of the outlet line of the GalliaPharm generator. Connect the bottom of the cartridge with a sterile needle.
j. Connect Vial 1 to the outlet line of the GalliaPharm generator by pushing the needle through the rubber septum and place the vial in a lead shield container.
k. Elute the generator directly into the Vial 1 through the cartridge and the needle according to the instructions for use of the GalliaPharm generator that are supplied by Eckert & Ziegler, in order to reconstitute the lyophilized powder with 5 mL of eluate. Perform the elution manually or by means of a pump.
l. At the end of the elution, disconnect the generator from Vial 1 by removing the needle from the rubber septum, and immediately (do not delay buffer addition more than 10 min) add the kit reaction buffer in the 1 mL sterile syringe (the amount of reaction buffer was determined from Step h). Withdraw the syringe and the 0.22 micron sterile air venting filter, and then using a tong, move Vial 1 to the heating hole of the dry bath, and leave the vial at 203 °F (95 °C, not to exceed 98 °C) for at least 7 minutes (do not exceed 10 minutes heating) without agitation or stirring. Do not invert or shake the reaction vial because contact between the solution and the rubber septum can lead to zinc leaching and can interfere with binding of Ga 68 to the peptide.
m. After 7 minutes, remove the vial from the dry bath, place it in an appropriate lead shield and let it cool down to room temperature for approximately 10 minutes.
n. Assay the whole vial containing the Ga 68 dotatate injection for total radioactivity concentration using a dose calibrator and record the result.
o. Perform the quality controls according to the recommended methods in order to check the compliance with the specifications [see Dosage and Administration (2.5)].
p. Prior to use, visually inspect the solution behind a shielded screen for radioprotection purposes. Only use solutions that are clear without visible particles.
q. Keep the vial containing the Ga 68 dotatate injection upright in a radio-protective shield container at a temperature below 77 °F (25 °C) until use.
r. After addition of Ga 68 chloride to the reaction vial, use Ga 68 dotatate injection within 4 hours.
s. Drug name 68Ga-DOTA-OCTREOTATE

Return to the Table of Content
12/17
Suggested reading, Theranostics in nuclear medicine practice, Yordanova A, et. al., OncoTarget and Therapy. 2017
References
1. Cyclotron production of 68Ga via the 68Zn(p,n)68Ga reaction in aqueous solution by Pandey MK, Byrne JF, et al. Am J Nucl Mol Imaging . v.4(4); 2014
2. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE by Virgonlini I, Ambrosini V, et al. Euro Journ of NM and Mol Imaging Vol. 37.10 Oct 2010