Nitrovasodilators
|
Sections |
| Structure |
| Metabolism |
| Biochemical Mode of Action |
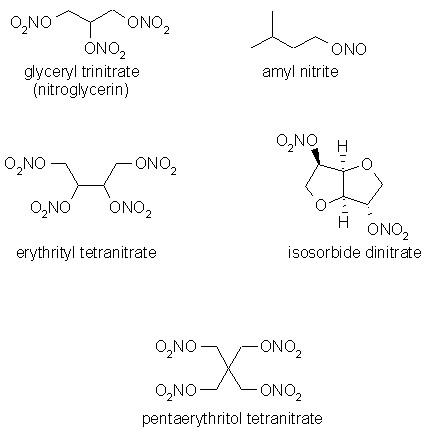
Nitrovasodilators are molecules that are esters of simple organic alcohols or polyols with nitric acid. This class was developed after the anti-anginal effect of amyl nitrite, an ester of isoamyl alcohol with nitrous acid, was first observed in 1857. There are five members of this class of anti-anginal drugs in clinical use today.

To view their three-dimensional structures click on the appropriate link. (Highly Recommended)
Except for amyl nitrite, all are nitrate esters (R-ONO2). The common name nitroglycerin may suggest that it contains a C-N bond due to the nitro word, however, the name is a misnomer. The molecule is a nitrate ester and is more correctly called glyceryl trinitrate. Another misnomer is amyl nitrite. The organic group is more correctly refered to as isoamylnitrite.
The chemistry of these molecule is easily predicted based on the structures presented above. i) These are small molecules; ii) they all are non-polar; iii) they are ester derivatives with susceptible C-O bond; iv) they are nitrate esters.
I) and II) lead to increased ease of vaporizability. Thus these are volatile drugs and may cause a problem is compounding. This singular property makes these drugs very helpful in emergency situations in which rapid absorption and action is essential.
III) leads to ease of hydrolysis. Thus moisture should be avoided to minimize the loss in principal active component.
IV) suggests that these compounds may be explosive, especially in pure concentrated form. Thus these compounds should be packaged in variety of diluents with excipients.
The structure also determines the onset and duration of action of these organic nitrates.
| Onset of Action (min) | Duration of Action (min) | |
| Amyl nitrite | 0.25 | 1 |
| Nitroglycerin | 2 | 30 |
| Isosorbide dinitrate | 3 | 60 |
| Erythrityl tetranitrate | 15 | 180 |
| Pentaerythritol tetranitrate | 20 | 330 |
Although the number of nitrate ester groups may vary from two to more than six, there is no direct relationship between the number of nitrate groups and the level of activity.
It appears that the higher the partition coefficient of the drug, the greater the potency. The orientation of the groups within the molecule also affect potency. The higher the lipophilicity of the compound the longer the vasodilatory response.
Organic nitrates are metabolized rapidly after oral administration by the liver, kidneys, lungs, intestinal mucosa, and the vascular tissue. Organic nitrates, nitrites and nitroso compounds generate nitric oxide (NO) in situ that forms the basis of their pharmacological action. The mechanism of release of NO from nitrites and nitrates is not clear.
Biochemical Mechanism of Action
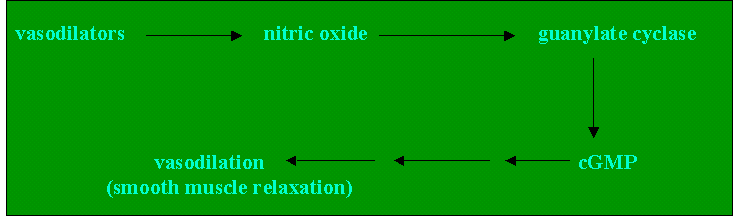
Although the physiologic effects of the organic nitrates are clearly due to its vasodilating effect on the vascular smooth muscles, the underlying molecular mechanism is not clear. Ignarro and co-workers proposed that nitrates act indirectly by stimulating the enzyme guanylate cyclase, thereby producing elevated levels of cyclic guanosine monophosphate (cGMP). It is suggested that nitrovasodilators undergo metabolic transformation in vascular smooth muscle cells to form NO. Nitric oxide mediates smooth muscle relaxation by activating guanylate cyclase.
Cyclic GMP so formed activates protein kinases that can regulate free Ca+2 levels in the muscle cell and cause relaxation of the smooth muscle by phosphorylating myosin light chain kinase (MLCK) a critical protein.
[top] [session home] [home] [school of pharmacy] [department of medicinal chemistry]
©2000 VCU School of Pharmacy
Revised: January 8, 2000
Questions or Comments : Dr. Umesh R. Desai