The LVCC method is based on coupling laser vaporization of metals with controlled condensation from the vapor phase. This setup consists of a modified upward thermal diffusion cloud chamber (DCC). The detailed description of the chamber can be found elsewhere. The chamber consists of two horizontal stainless steel plates separated by a circular quartz ring. A target, which can be made of a pure metal or a mixture of metallic powders, is placed at the center of the bottom plate of the chamber. Immediately after the laser beam hits the target, a shock wave was initiated by the collisions between the gas and the target atoms and a plume is emitted. The evaporated metal atoms collide with the inert gas atoms at the front of the expanding plume. As a result, plume atoms rapidly thermalize in 10-100 microseconds after the laser pulse. The degree of thermalization required for condensation is related to the vapor pressure of the target material and the efficiency of the energy transfer during gas-target atom collision depends on their atomic masses. The cooling plume confined behind the shock wave becomes supersaturated, leading to nanoparticles formation via homogeneous nucleation. The temperature gradient, as well as the high static pressure inside the chamber helped in creating a steady state convection current which carried the generated nanoparticles to the top plate, where particles deposit. The higher the convection rate inside the chamber, the faster the particle can be removed from the nucleating zone (plume generated by the laser) and the smaller the average particle size distribution.
Laser Vaporization Controlled Condensation
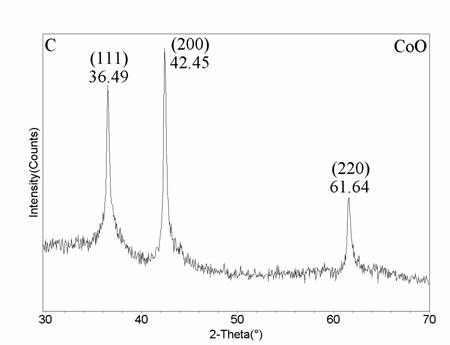
Herein, we report for the first time the synthesis of cobalt nitrate hexahydrate, cobalt oxide, and cobalt particles formed from a high purity cobalt metal by a novel laser vaporization controlled condensation (LVCC) method under controlled pressures of N2 and O2. The metal vapor produced from a cobalt target in the presence of 50% N2 and 50% O2 results in the formation of cobalt nitrate. We also explored the possibilities of forming cobalt oxide and cobalt nanoparticles by altering the ratio of N2 and O2 present. For example, the synthesis of pure cobalt oxide (CoO) nanoparticles is of importance and challenging since a simple chemical route is complex. We believe that this work will be of significant importance since the present method is promising for the synthesis of metal mono oxides.
Full Story