131I-meta-iodobenzylguanidine (mIBG) Therapy
- Pathology
- These tumors develop from primitive neural crest, which is part of the sympathetic nervous system and is associated with the neuroendocrine system
- These cells are sometimes referred to as APUD cells - (amine precursor uptake and subsequent decarboxylation)
- APUD produce amines, polypeptides, and steroids which, in part, regulate "normal homeostatic mechanisms within the body," vasomotor tone, carbohydrates, calcium, and electrolytes"
- Characteristics of mIBG
- Classified as a noradrenaline or norepinephrine (NE) analog and are defined as a false neurotransmitter
- APUD cells have an affinity for mIBG. There are two pathways of uptake
- Uptake one - is an active process were neuroblastoma cells have a high affinity for NE analog mIBG (this is the main cause for visualization)
- Uptake two - uptake occurs through passive diffusion
- Once mIBG enters the cell, it is stored in neurosurgery granules (pheochromocytoma cells) and/or stored in the cytoplasm and mitochondria of neuroblastoma cells. mIBG does not breakdown and is not utilized in adreneregic receptors. In essense, it gets "locked in"
- Applying 131I to mIBG allows for treatment of certain neuroectodermal tumors: pheochromocytoma, paraganglioma, carcinoid, medullary thyroid cancers and neuroblastoma
- The radioiodine delivers ~ninety percent of the beta particles containing 606 keV of energy and travels about 0.5 mm in human tissue
- Therapy should not be done in the following situations
- Patient is pregnant or breast feeding
- Life expectancy is <3-months, however, if this person has painful bony mets, this treatment may relieve so of this discomfort
- Renal insufficiency - the patient would require dialysis if this therapy is applied
- Myelosuppression
- <3000 WBC
- < 100,000 platelets
- Patients with reduced white cell or platelet count or poor renal function may still receive treatment, but at a lower dose
- Clinical Trials
- Several clinical trials have been done to evaluate the use of a therapeutic dose of mIBG
- In 1991 a Phase 1 trial had 14 patients that received 200 mCi of 131I to mIBG with a zero % response rate
- Then in 2007 (Phase 2) 164 patients received 18 mCi/kg of 131I to mIBG and had a 36% response rate with 13 patient having complete remission
- Bone/bone marrow involvement had 45% involvement
- Soft tissue had 50% involvement
- Stem cell involvement occurred in several therapies cases which included chemo and radiation therapy. This causes complete destruction of the stem cells requiring stem cell transplant
- Combining 131I to mIBG with chemotherapy has caused venous occlusive liver disease (VOLD or VOD). One study reported 6 out of 22 patients acquired VOLD that has some correlation with patients that have poor GFR5
- Protocol
- Patient preparation and requirements
- Neuroendocrine tumor staged at III or IV and acquisition data from a 123I-mIBG scan prior to therapy.
- Tumor must be mIBG avid prior to therapeutic administration. MRI, CT, or Ultrasound are also required
- Dose with iodine supplement two days prior and 10-15 post treatment
- There are many types of medications that can interfere (with its uptake or retention) when mIBG is administered - list
- Catecholamine secreting tumors (PCC) may receive medications that are labeled as alpha and beta blockers. This helps control the disease. These medication interferes with mIBG uptake and must be discontinued prior to therapy. Note - withdrawal symptoms may occur when discontinuing alpha and beta blockers
- Administration of mIBG may cause hypertension and for this reason the dose must be administered slowly
- Pediatric patients may require additional care and parent(s) taking care of the child should undergo radiation safety training. Consider these exposure levels to the "public," no more than 100 mrem per dose or a total of 500 rem NRC 20.1301
 3
3
- Administering the dose
- Patient should be placed on anti-emetic medication prior to the exam and up to 72 hours post dose
- Dose is usually 18 mCi/kg and may be administered over 45 minutes adn up to 4 hours4
- The patient should be monitored with a concern of possible onset of hypertension
- Catecholamine secreting tumors may also cause hypertension requiring the use of short-acting alpha and beta blockers
- In particular situation, the infusion may be slowed or stopped
- F. Giammarile, et al. identifies the administering dose to be between 100 - 300 mCi1
- Several therapeutic doses can be administered to a single patient over a period of time. It depends on the patient's response to treatment
 3
3
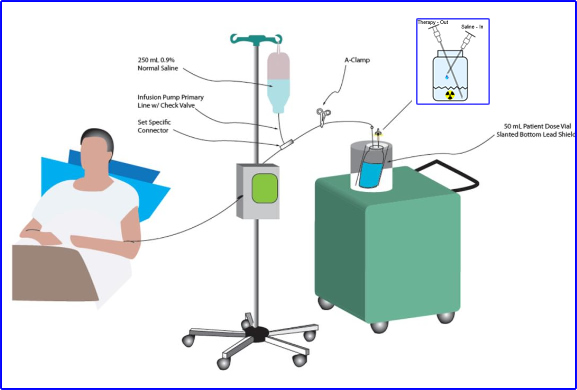
- Look at the dosing vial
- Example shows how a vial of 131I to mIBG is shielded
- Other points of interest
- Nursing personal and family caregiver should be given instructions on radiation safety
- The patient should drink lots of fluids and urinate frequently to reduce the radiation burden to the urinary bladder
- Radio urine is a concern for up to five days post dose (+)
- The patient should flush the toilet x2 per visit
- Incontinent patients require foley catheterization
- Myelosuppression must be monitored after the therapeutic dose
- Imaging during treatment may yield additional assessment
- Side effects
- Nausea and vomiting may occur within the first two days post therapy
- Myelosuppression usually occurs between 4 to 6 weeks post treatment and chemotherapy may further complicate the suppression
- Bone marrow involvement will add to the myelosuppression
- Rarely renal function can be effected, however, there is an increased concern when specific chemotherapy agents are used with mIBG therapy
- Thrombocytopenia and extended myelosuppression may be a concern
- Rarely leukemia can develops
- Beware of VOLD
- Hypothyroidism has been reported and it appears to be an issue even with the use of iodine supplement
- One researcher reported secondary malignancies to occur ~5%
- Results
- Relationship between dose, remission, and pain
- Research indicates that lower therapeutic doses result in few remissions of tumor
 3
3
- Lower doses appear to have a greater effect on reducing pain associated with the disease. In this case, a pediatric patient received 4.9 mCi/kg and became pain-free in her left leg
- Several repetitive 131I mIBG treatments yielded the following results:
- Fourteen days between two therapies (no chemo) with replacement of hematopoietic cell transplantation (HCT) to offset myelosuppression. The dose was 50 mCi/kg. There was a 45.5% positive response rate when the disease was in the soft tissue. However, bone involvement only had a 15.4%.6
- Patients receiving 18 mCi/kg with a 42 to 100 days between the doses had a 39% positive response rate7

- Patient with malignant pheochromocytoma received a high dose of 131I to mIBG. The exact amount or the amount of times of the therapy administered was not stated in the article.8
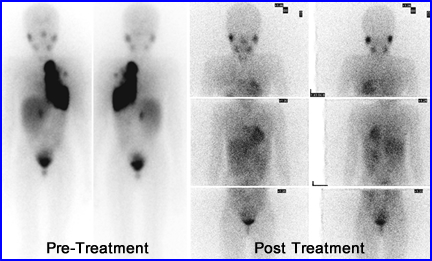
- This is a seven year old female with stage IV neuroblastoma. She received 300 mCi of 131I to mIBG. These authors also stated that tumors treated with radiotherapy only have a 32% response rate, while combined radiotherapy and chemotherapy had a 39% response rate.9

- Imaging assessement - Comment on this image
6/21
Return to Table of Content
1- Reference - EANM procedure guidelines for 131I-meta-iodobenzylguanine (131I-mIBG) Therapy by F. Giammarile, et al. (2008)
2 - Kayano D and Kinuya S. Iodine-131 Metaiodobenzylguanidine Therapy for Neuroblastoma: Reports So Far and Future Perspective. ScientificWorldJounal. 2015: 189135
3. I131-mIBG Therapy for Neuroblastoma PPT presentation by Meaghan Grander, MD. Cook Children's
4. Package insert - https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209607s000lbl.pdf
5. Matthay KK, et al Phase I dose escalation of iodine-131metaiodenzylguanidine with myeloablative chemotherapy and autologous sem-cell transplantationin refractory neuroblastoma: a new approach to neuroblastoma therapy corsortium study. Journal of Clnical Oncology. 2006;24(3):500-506.
6. Clement S. C., et al. Long-term follow-up of the thyroid gland after treatment with 131I-Metaiodobenzylguanidine in children with neuroblastoma: importance of continuous surveillance. Pediatric Blood and Cancer. 2013;60(11):1833–1838.
7. Johnson K., et al. Safety and efficacy of tandem 131I-metaiodobenzylguanidine infusions in relapsed/refractory neuroblastoma. Pediatric Blood and Cancer. 2011;57(7):1124–1129.
8. Eisenhofer G, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Society of Endocrinology 2004;11(3): 423-436
9. Piccardo A, et al.131I to mIBG Therapy of Malignant Neuroblastoma and Pheochromocytoma. Nuclear Medicine Therapy pp 65-83
3/21
 3
3 3
3

 3
3

