- This is the physical decay of a radionuclide
- It is defined as the time in which it takes number of atoms to disintegrate to ½ the original amount
- The rate of decay remains constant and cannot be altered, even with the application of temperature, pressure, or chemical interaction
- As a radionuclide decays over time fewer of the original atoms remain. This remaining fraction of atoms, that decay per unit time, is known as the decay constant (λ)
- The larger the λ per unit time the shorter the half-life (T½)
- This has an inverse relationship
- While the literature refers to λ as being “constant” in reality it is a statistical value, which means that there maybe a slight variation in the decay time
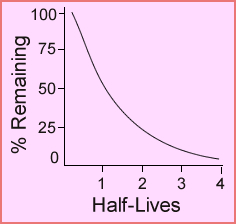
- Refer to the chart below an note that the y-axis represents the number of radioatoms and the x-axis represents the number of T½
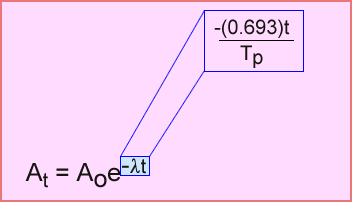
- The decay formula can be expressed in the following formula.
- At = number of atoms at a certain point in time
- Ao = number of atoms originally present
- e = base of the natural logarithm, 2.718
- λ decay constant
- t = time
- Expanding “– (λt)” the formula can be re-written. Note 0.693 is the natural log of 2
Let’s apply the decay formula to see how this works!
Question: You have 100 mCi of 99mTc. How much activity remains in 2 hours?
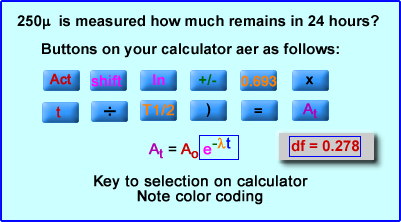
These entries are used on scientific calculator (TI-30 IIS)First calculate e-λt
- Enter 100
- Hit the 2nd or shift key
- Select the ln button (e)
- Select the +/- button
- Type the natural log of 2 which is " 0.693"
- Now enter the time (t), "2" (hours)
- Then choice the T1/2 "6.02" (hours)
- (This generates a decay factor, e-λt which is 0.794 )
- Enter parenthesis - ")"
- Equal sign "="
- At = "79.4" mCi
Another approach to decay is the formula below which allows you to decay into the future. If you want to decay into the past make the exponent negative
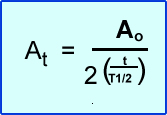
- Either way you calculate this your answer will still be same
- It is up to you to decide which method you wish to apply when calculating decay
- Determine the amount of activity remaining in a vial a 123I if the initial capsule contained 221 μCi at 12N. How much remains at 8:30am the following morning. Answer
- 99mTc has 1.0 mCi of activity. Decay this every half-hour, up to 6 hours . Put this information on a separate piece of paper and place that data on a chart, showing time elapsed and the amount of activity remaining. Answer
- If you had 100 mCi of 131I today (12N), how much did you have two weeks ago (at 12N) Answer
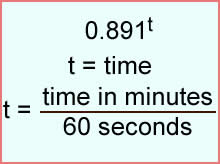
- t = time
- Time is s ratio of the amount of minutes into the future divided by 60 minutes
- Assume you have 100 mCi at 7am, how much activity remains at 8:41? Answer
- This is the time it takes the body to eliminate ½ of the radioactive material
- It has nothing to do with Tp, however, it is related to Tp (more on that in a moment)
- Methods in which the body eliminates a radioactive compound would include: urine, feces, perspiration, and/or exhalation
- When related to the Tp the biological effects of a radioactive compound is limited if the Tb is very short (even when the Tp is very long)
- When combining the Tp and Tb the result is Te
- Will allow you to calculate amount of radiation absorbed by the body or even a specific organ
- If Tp and Tb are known then the following formula can be used to calculate Te
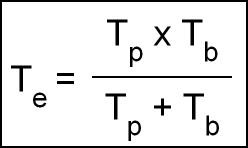
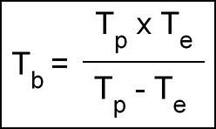
- 99mTcMDP
- 123I
- 99mTc sulfur colloid