- There are 118 known elements, 94 of which are believed to occur "naturally"
- Atoms are composed of
- Electrons (e-)
- Negatively charged
- Has an energy of 1.6 x 10-19 coulomb
- Mass of 0.9109 x 10-27 g
- ~1/1837 AMU
- Protons (P+)
- Positive charge
- Mass of 1.6726 x 10-24 g
- ~1 AMU
- Neutrons (No)
- Neutral charge
- Mass of 1.6747 x10-24 g
- ~1 AMU
- Notice the AMU difference of an electron to that of a proton or neutron
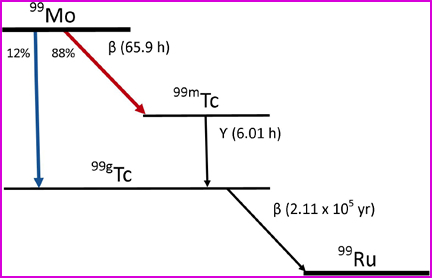
- In nuclear medicine the Tri-Linear Chart has a more definitive purpose as it gives essential information about decay/half-lives/emitters/energies
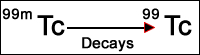
- Look at 99Mo and 99mTc
- What is being emitted?
- What are their energies?
- What are their T1/2?
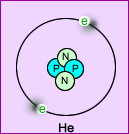
- Atomic structure
- In the center of an atom are protons and neutrons
- Orbiting around this structure are electrons
- For every proton in the nucleus of an atom there are an equivalent number of electrons
- Remember that ionization occurs where there is a loss of an electron. How might that happen in nuclear medicine as it relates to radiation?
- The element of an atom is expressed by the number of protons in the nucleus (This is also know as the Z number)
- X represents the type of element
- Z, is referred to as the atomic number which is/are the number of protons in the element
- A, the mass number represents the number of neutrons and protons present in the atom
- While the size of an atom does vary the range is between 1 to 2 10-8 cm
- The size of the nucleus of an atom is significantly smaller (10-13 cm in size)
- The relationship between the protons and the electrons
- The amount of P+ in an atom identifies the element
- The amount of P+ = to the amount of e- orbiting the nucleus
- Coulomb force (electrical force) occurs between the P+ and the e- and provides stability in this relationship
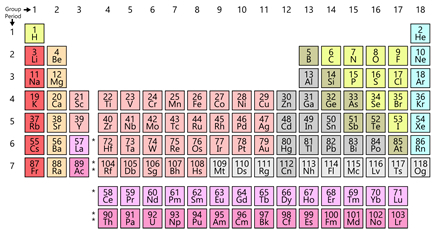
Click Periodic Table to enlarge - which elements are radioactive?

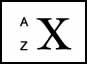
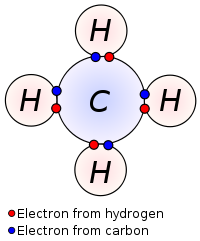
- Formed by two or more atoms (ex. CO2)
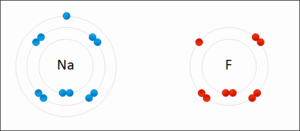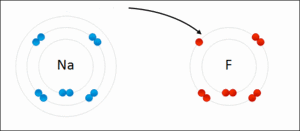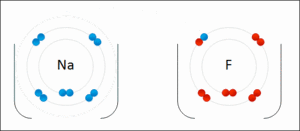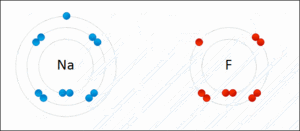
- In order to form a molecule the following must be considered
- Binding of two atoms occurs with the outer shell electron (also known as the valence electrons)
- Types of bonds
- Ionic - Sodium Fluoride
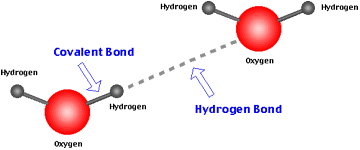
- Covalent bond - Hydrogen
- Hydrogen bonds a type of covalent bond
- Chemical Party of the elemnets on You Tube
- Many of nuclear medicine radiopharamacetuicals have a covalent relations ship with the radioactive atom - Here is an example
- An isotope is defined as an atom or element having more than one form. The variation occurs with the amount of neutrons (A number varies), but the amount of protons remain the same (Z number)
- Good example where this shows up nuclear medicine are with the iodines (123I, 124I, 125I, 127I, and 131I)
- Which of the above iodines is non-radioactive?
- An isobar is defined as two atoms that have the same mass number (A), but the different atomic numbers (Z). Why are they different elements?
- Isotones are nuclides that have the same neutrons, but vary in the number of protons, hence the Z number and A number are differ. They are also different elements
- An isomer is a nuclide that has the same mass number, atomic number, and element, but it is in an excited energy state. This excited state may last as little as 10-12 seconds or longer. The excited energy state will eventually cause the emission of a gamma ray. In Nukes 99mTc is very important and is the radioactive component used in many of our radiopharmaceuticals
- Of the Iso_s mentioned, in nuclear medicine Isomers and Isotopes are of the greatest importance
![]()

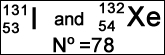
- In order to maintain the neutrality of an atom there needs to be a balance between protons and electrons. When an element has too many and of either it will decay
- Consider the differences between the mass of an electron, proton, and neutron
- When all electrons fill an electron shell it is very stable and known as an inert element (ex. in nuclear we use 133Xe)
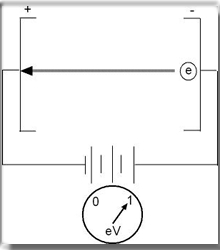
- Is a unit of measurement used in nuclear medicine. Usually we refer to this measurement in meV or keV
- 106 eV = 1 meV
- 103 eV = 1keV
-
Electron volt is defined as the amount of kinetic energy required for an electron to fall through a potential difference of one volt (V). Hence, if 1 electron passes through a potential difference of 1 V then this is = to 1 eV. However, if 106 electrons fall through a potential difference of 1V then the this would = 1 meV