- Therapeutic Moab used to treat patients with non-Hodgkin's lymphoma.
- Usually the course of this therapy is used when other treatment regimens have failed and the disease is still considered life-threatening
- Ibritumomab is a murine IgG 1 molecule kappa Moab
- Attaches to the surface of the CD 20 Antigen which is found on the surface of normal and malignant B lymphocytes
- The entire IgG molecule is used in this procedure so expected a long clearance time
- Heavy chain is composed of 445 amino acids
- Light chains are composed of 213 amino acids
- Comments on the diagram: Blue timeline indicates imaging and purple is for therapy. This procedures requires two protocols for completion.
- Dosing and imaging the Moab distribution (Part 1) and dosing the therapeutic (Part 2)

- Part 1 - The imaging component ( 111In ibritumomab tiuxetan) initial distribution determines whether or not the therapeutic moab can be administered
- Rituximab (non-radioactive Moab) is given to the patient prior to any radioactive Moab administration
- Binding of the radiotracer without the cold Moab will cause an 18% increase in the binding of the radiotracer invivo
- If non-radioactive rituximab is given prior to the radioactive agent the binding of ibritumomab increases 92%
- Following the dosing of Rituximab, within four hours, 5 mCi of 111In ibritumomab tiuxetan must be administered
- After administration of the radioMoab, usually two scans are acquired (however, a third maybe done if there are questions that cannot be answered from the first to scans
- Timeline for imaging
- First scan: 2 – 24 hours post dose
- Second scan: 48 to 72 hours post dose
- Third scan: optional and may be done up to 120 hours
- Normal distribution is as follows:
- 2 – 24 hours after injection intense uptake will be noted in the vascular pool
- Forty-eight hours after the injection a reduction of vascular activity is noted. Additionally, there should be significant distribution within the liver and spleen. Low to very low uptake is noted in kidney, urinary bladder, and bowel
- Radiotherapy agent will not be administered if
- Uptake is seen greater in the lung than the heart on the initial images or
- More activity in the kidneys vs. liver/spleen (post 48 hours) or
- Greater uptake in the large bowel when compared to liver/spleen (post 48 hours)
- Abnormal uptake prevents the administration of the radiotherapy; however, this rarely occurs (less than 1%)
- Two tables in this lecture (below) further define normal and abnormal uptake of Indium.
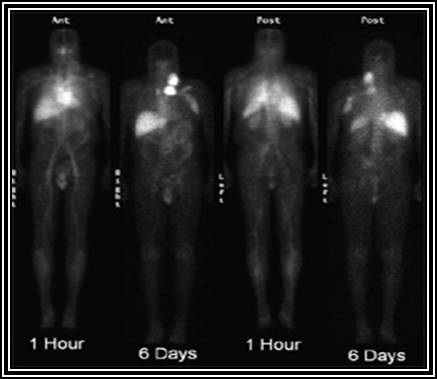
The above images show normal distribution of 111In ibritumomab tiuxetan. The uptake is within normal limits and allows for the administration of 90Y ibritumomab tiuxetan. Note the increased activity in the area of non-Hodgkin lymphoma.
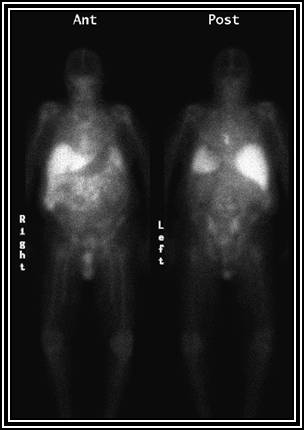
This is a another second example of Indium-Moab uptake at 92 hours post administration. No abnormal uptake is noted therefore, radioimmunotherapy (RIT ) will continue on day 7 (see timeline - chart).
- Part 2 - 90Y ibritumomab tiuxetan administration
- Unusually given between the 7th to 9th days into the initial timeline
- Note the similarities in protocols between the 111In agent and the 90Y agent
- It should be noted that patients are treated as outpatient since the amount of the administered dose is below what the NRC would require for hospitalization
- Prior to the therapeutic dose
- 250 mg/mL of Rituximab is given
- The therapeutic dose must be administered within four hours
- The decision to dose 90Y ibritumomab tiuxetan is based on platelets count
- Platelet counts between 100k – 149k/μL = 0.3 mCi/kg
- Platelet counts greater than or equal to 150k/μL = 0.4 mCi/kg
- Counts below 100k/μL are not treated
- Should be injected over a 10 minute time frame
- Biodistribution
- Half-lives
- Median effective T ½ = 28 hrs (14 – 36 hrs)
- Median biological T ½ = 48 hrs 18 – 77 hrs)
- Injection of rituximab (retuxan) depletes the body of the B lymphocytes in circulation. This allows for targeting CD 20 antigen when diagnostic and therapeutic agents are injected, thus improved tumor uptake of the radio-Moab
- Tumor uptake is usually seen but is not required for administration of the therapy agent
- Half-lives
| Scan 2 – 24 Hours | Scan 48 – 72 Hours | Scan 90 – 120 Hours | |
|---|---|---|---|
| Vascular Pool | Seen | Decreased | Decreased |
| Liver/Spleen | Moderate to high | Moderate to High | Moderate to high |
| Renal, U. Bladder, Bowel | Low to very low | Low to very low | Low to very Low |
| Tumor | Variable | Variable | Variable |
Altered Biodistribution - 111In ibritumomab tiuxetan
| Scan 2 – 24 Hours | Scan 48 – 72 Hours | Scan 90 – 120 Hours | |
|---|---|---|---|
| Vascular Pool | Not Seen | Not Seen | Not Seen |
| Lungs | >Cardiac Blood Pool | Diffuse uptake > liver | Diffuse uptake > liver |
| Renals | > Liver in POST | > Liver in POST | > Liver in POST |
| Bowel | > Liver | > Liver | > Liver |
- Other comments
- Toxic effects include transient hematological toxicity which is usually reversible
- Labeling and administration the diagnostic and therapeutic agents
- 111In ibritumomab tiuxetan
- Make sure that all contents are at room temperature before compounding (storage of the Moab is 2 – 8o C)
- Sodium acetate (50 mg/mL) is initially placed into the reactive vial at 1.2 times the volume of the Indium tracer
- 5.5 mCi of 111In chloride is then added
- Ibritumomab tiuxetan (1.6 mg/mL) is added (1.0 mL total)
- Gently mixed at room temperature for 30 minutes
- After incubation immediately add buffer to bring the compound up to 10 mL
- In a 10 mL syringe with 18 to 20 gauge needed withdraw the required amount of radio-Moab
- Placing an IV into the patient and administer the tagged Moab over a 10 minute timeframe using a shield syringe pump (note – the cold Moab has already been administered)
- 90Y ibritumomab tiuxetan is given after the imaging the Indium agent. Therapy should occur between 7 – 9 days post diagnostic Moab. Note variations in the therapeutic administration of 90Y preparation
- 40 mCi of 90Y chloride is placed into the reaction vial
- Sodium acetate 50 mol/L is added which must be 1.2 times the 90Y volume
- The amount of Ibritumomab tiuxetan 1.3 mL
- Compounding order the same
- Note the correct order of compound for both procedures: sodium acetate, radionuclide, and then the Moab
- Incubation time is only for 5 minutes
- Buffer amount is the same
- Dose is based on platelet count with a maximum dose being 32 mCi
- Administration is the same as the diagnostic Moab and must be done within 8 hours following compounding
- 111In ibritumomab tiuxetan
- Testing for radiochemical purity (RCP)
- Strip - ITLC-SG
- Solvent - 0.9% saline
- The tagged Moab is found on the bottom of the strip (B) and any free radionuclide climbs to the top (A)
- Using a single strip calculate - (B/B + A) x 100 = % RCP
- Using the dose calibrator
- 90Y is a pure beta emitter and requires specific adjustments to the dose calibrator
- Setting the calibrator on manual and dial in 48 is used with Capentec dose calibrators
- Order from the pharmacy a specific calibrated about amount of 90Y in a volume of mL syringe in a 10 mL syringe
- After placing the dose into the calibrator adjust your dial so that you get the same reading supplied by the central pharmacy. Note - the dose calibrator reading is multiplied by 10 because it is a required correction given by the manufacturer (your reading pure beta)
- Now add 1 mL aliquots of saline to the 10 mL syringe and record your activity for each mL increase
- Do this till you reach 9 mL
- You will have readings for 1, 2, 3, 4. 5, 6, 7, 8, and 9 mL
- Based on your 1mL reading determine the geometric variation for volumes 2 - 9 mL
- If the variation is greater than 2% you must determine a correction for that specific volume
- A worksheet has been place for your review at the following link Geometeric Variation
- The actual reading is 2.0 mCi
- The volumes being recording are 1 - 9 mL
- Where ever the dose varied from 2.0 mCi geometric variation was determined (4, 5, 8, and 9 mL)
- Correction factor was calculated and applied to the appropriate dose
- Results
of therapy
- Clinical trials (phase III) indicate a positive response rate to therapy. A total of 143 patients were dosed
- 80% responded to the therapy
- 34% were free of non-Hodgkin’s lymphoma at 23 months
- Adverse events(<5%) included but not limited to
- Infection
- Chills
- Fever
- Abdominal pain
- Hypotension
- Nausea
- Vomiting
- Clinical trials (phase III) indicate a positive response rate to therapy. A total of 143 patients were dosed
- Additional case presentations
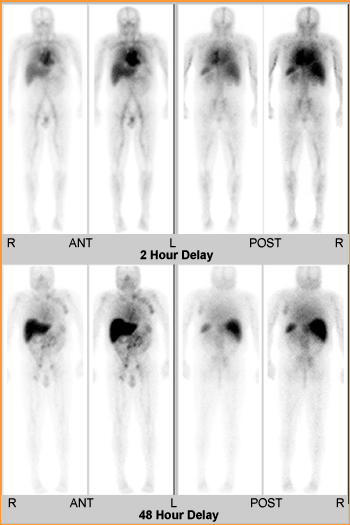
- Normal distribution of 111In-zevalin with adequate clearance from the soft tissues, demonstrating the patient's eligibility for the 90Y-zevalin treatment.
- Increased activity seen in the left axilla, bilateral iliac regions, and uptake of the zevalin in the mid abdomen.
- This case can be found at http://www.rad.kumc.edu/nucmed/clinical/zevalin2.htm
Additional Comment - there is a significant amount of paper work/documentation that goes along with a Zevalin procedure that was not be covered in class. In the clinic you will be required to identify and apply any/all paper work that was not covered in class.
Return to the Beginning of the Document
Return to the Table of Content
For more information about Zevalin visit SPECTRUM Pharmacaeuticals
Reference material from this lecture was supplied by Biogen Idec application representative. A reprint from Seminars in Nuclear Medicine was used Volume 34, Number 1 (Supplement 1). Yttrium 90 Ibritumomab Tiuxetan (Zevalin) Radioimmunotherapy for B-Cell Non-Hodgkin’s Lymphoma.