Bone Palliation by Radiopharmaceutical
- The use of Beta emitting radiopharmaceuticals for the relief of bone pain (palliative therapy).† Pain is do to metastatic bone disease
- Certain Beta emitting radiopharmaceuticals can be used for the relief of bone pain caused by painful bony mets
- What are the methods for relieving pain caused by bony metastatic?
- From a therapeutic standpoint metastatic disease is usually treated with either with chemotherapy and external beam radiation.
- One approach to managing pain is to apply external beam radiation to the site. However, over time metastatic disease tends to spreads. These increasing sites of pain make external beam therapy a less effective method
- The approach to external beam therapy has two roles: first it can shrink and/or destroy tumors and second it can be used to target painful bony mets for the relief of bone pain. The later approach can be rather ineffective in treating multiple/painful metastatic sites as those sites continue to multiple.
- Chemotherapy can be used for pain management however, excessive toxicity and multiple metastatic sites makes it difficult to continue this line of approach
- Pain medication, usually Opioids, is yet another method used to relieve bone pain, yet these narcotics have side effects, which would affect the patientís quality of life. These side effects include: constipation, gastrointestinal distress, sedation, nausea and vomiting, and cognitive dysfunction. These side effects can be especially noted with high levels of morphine
- An alternative approach is the use of beta emitting radiopharmaceuticals for the palliative treatment of bone bone: 89Sr or 153Sm
- Why should one use a beta emitter to treat bone pain do to metastatic bone disease?
- Ultimately, the best course of action, for most people with multiple metastatic sites is to treat the pain and not the disease
- There are several choices regarding the use of these beta emitting radiopharmaceuticals for the treatment of bone pain.†The historical perspective
- As early as the 1940s 32P-Orthophosphate was considered for the administration in patients that have metastatic bone pain. The hope was and is still, not to cure metastatic bone disease, but to relieve pain via a therapeutic injection of a radiotracer. This should releave the bone pain and reduction of the use of pain medication, allowing the patient to have a better quality of life
- Application of 32P during the 1940s
- This agent has been given to 1000s of patients with over 850 reports in the medical literature
- 85% of the dose is incorporated into the hydroxyapatite crystal of the bone; however, 15% is taken up by non-bone tissue and here is where the problems occur. Phosphate involves itself, intracellular with energy storage, cell structure, and in DNA\RNA structure (soft tissue)
- In turn, the beta radiation destroys the nucleic acids, because it †incorporates into these structures. Therefore, there is additional radiation effects to the body, that may not occur with the newer radiopharmaceuticals that will be discussed
- Primary cancers, that originate from breast and prostate cancer metastasize to bone are generally considered blastic lesions
- These blastic lesions involve osteoblastic bone cells. When metastatic disease invades the bone an increase in calcium deposits occurs at the site. Performing a routine bone scan in a nuclear medicine department can identify these sites. It is this type of lesion that are effected by the use of our therapeutic radiopharmaceuticals
- On the other hand lesions that are not recommended for radionuclide therapy are those of a lytic nature. Lytic lesions proliferate from neoblastic plasma precursor cells in bone marrow and bone pain originating from this type of lesion is not effective by our beta producing radiopharmaceuticals.
- Hence, palliative therapy should only done on those patients in which blastic lesions. The two most common metastatic diseases that are recommended for treatment are breast and prostate
- How do these lesions cause bone pain?
- Although the exact reason for metastatic pain bone is unknown, several possibilities have be theorized
- The primary cause of bone pain is thought to be an inflammatory process associated with the metastatic invasion of disease into normal bone tissue. When this occurs it changes bone metabolism resulting in autocoid production. These process has been seen with blastic and lytic lesions
- Autocoids are heterogeneous group of chemicals released by the body in response to tumor invasion. Some of these substances include cytokines, potassium, bradykinin, growth factors, and osteoclastic activating factor. Osteoclastic activity can further stimulate prostaglandin production
- Therefore, in theory the production of autocoids and prostaglandin cause an increase in stimulus to the nerve ending within bone, which results the pain
- Also through direct tumor invasion to a nerve or in the extensive bone destruction of bone different types of fractures can occur (including compression) which results in severe pain
- So how does 89Sr and 153Sm fit into the therapeutic picture?
- Uptake by bone depends on the metabolic activity within the bone, the rate of new tissue formation, and tissue vascularity. Hence, uptake of a therapeutic radiopharmaceutical depends on bone turnover within the bone/tumor, its vascularity, and the rate of new tissue formation.
- It is unknown as to the exact reason for these agents to relieve bone pain. However, in theory these radiopharmaceuticals do incorporate into the bone matrix and hence allow for direct radiation to sites of high bone turnover. The highly energetic beta particle deposits its radiation close to the metastatic tumor cells. Likewise, the greater the uptake of the specific radiopharmaceutical the greater the radiation to the tumor site. The result is relief of bone pain
- 89Sr Chloride
- As an analog to Ca it binds to the hydroxyapatite molecule
- The ratio of strontium uptake in active bone tissue surrounding a metastatic tumor is between 15 - 3 to 1
- The biological T1/2 is 4-5 days, with the body eliminating 2/3 via kidneys and 1/3 via the bowel. Of the initially deposited tracer 30 to 35 percent remains in the bone at 10-14 days post administration with 20% remaining after 3 months
- Sr-89 has a physical T1/2 of 50.5 days with maximum beta energy of 1.43 meV and a mean tissue range of 2.4 mm. It also has a 910 keV gamma, however the abundance is only 0.01%. The lack of significant gamma abundance dose not makes Sr a candidate for scintigraphic imaging
- Myelosuppression can occur with the use of this agent which usually causes 60 to 70% drop of platelets and WBCs at 5 to 8 weeks post administration, with recovery between 10 to 16 weeks post administration
- Flare reaction may occur in the patients with a temporary increase in bone pain. If it happens it usually occurs between 2 - 3 days post injection.
- Overall relief from the initial bone pain appears in as little as 3 days post injection, with a more common response by the second week. In some patient it has taken up to 25 days. The published data indicates that 65 to 80% of all patients receive some relief of pain, while 5 to 20% have complete relief bone pain. Pain reduction usually lasts between 3 to 6 months. Re-administration of Sr-89 maybe done every 3 months, with about half of these patients responding to treatment
- 153Sm-EDTMP
- 153Sm chelates to EDTMP (similar properties to MDP) that forms an insoluble oxide on the hydroxyapatite surface, yet it clears even faster than MDP, with renal excretion completed at 8 hrs post administration. There is <1% remaining in the vascular pool at 5 hours. Animal studies indicate that less than 0.5% binds to serum protein.
- 65% +/- 15% of the total dose goes to the bone. This percentage increases as bone turnover increases
- Sm has a physical T1/2 of 1.9-day and emits 3 different energy betas with the average beta energy at 233 keV. These beta particles travel 1.7 mm in bone and 3.1 in soft tissue. There are 2 gamma energies admitted at 103 and 41 keV, which allowing for sctinigraphic imaging
- The above is an example of a bone scan taken on a patient just prior to his therapy treatment of 153SmEDTMP
- Patient hydration is important which helps in renal excretion further reducing the radiation burden to the kidneys and bladder.
- Flare reaction occurs in approximately 7% of the patients at 2 to 3 days post administration. 75% of the radiation doses to the effected sites are delivered in 4 days post administration while myelosuppression occur between 3 to 5 weeks post administration via a drop in platelets and WBCs. Normal function returns at 8 weeks post administration

http://db.doyma.es/cgi-bin/wdbcgi.exe/doyma/mrevista.fulltext?pident=13052919 - The efficacy of 153SmEDTMP or Quadramet was determined from two clinical trials in which a total of 270 patients were given either Quadramet or a placebo.
- Trials were randomized, double blind, meaning that the physicians did not know anything about the patients.
- Patients were also graded twice a day on his/her level of pain with a ranged of 0 (no pain) and 10 (excruciating pain). The level of oral morphine was monitored in its mg value and the weekly average dose was determined. The morphine intake was then calculated with patients that received Quadramet and the placebo
- It was determined that relief of bone pain was greater with those individuals that received Quadramet which showed a reduction in pain with a reduction of morphine administration. This occurred 1-week post administration. The greatest relief of pain occurred between 2 to 5 weeks post administration
- In the analysis 399 patients, 23 deaths occurred where the average occurrence was on day 67 post administration. While 46 patients had serious events reaction to the therapy agent could not be confirmed. It was believed that was more of the results of the underlining disease and had no association with Quadramet
Procedure
- Patient Preparation
- Obtain a recent complete blood count
- White blood cell count should be greater <3,000
- Platelet count should be greater than < 60,000
- Review the procedure with the patient Explaining the procedure to the patient and have them sign a consent form
- Discuss radiation safety issues to the patient's family and the general public. Sm-153 has a significantly greater dose
- Patient cannot be sensitive to phosphonates if 153SmEDTMP is administered
- If the patient is incontinent, he/she should be catheterized
- Have the patient sign a consent form
- Setup an indwelling catheter with saline flush prior to administration
- Radiopharmaceutical Dose
- For 89Sr the average dose is is 4.0 mCi, however, a 40 to 60 :Ci/kg dose of body weight can be used
- For Sm-153 EDTMP the dose should be calculated at 1.0 mCi/kg of body weight. Note - a patient that weighs 70 kg will require a 70 mCi dose
- Use a syringe shield must be modified with plastic syringe for beta radiation. However, with the use of 153Sm lead or tungsten should be considered
- Administration Technique
- After setting the indwelling catheter attach a three-way stopcock to the IV line
- Insure that the needle is properly in the vein prior to injecting the radiopharmaceutical
- Administer the radiopharmaceutical over a 1-minute
- Flush the syringe with a saline bolus and turn up the IV drip to further flush the line
- Additional Comments
- Regulation Guide 8.39 allows the patient to be discharged, even if the dose is greater than 30 mCi
- You may want to follow the patient platelet and WBC counts after radiopharmaceutical administration
Comparison of 89Sr and 153SmEDTMP
Sr-89 |
Sm-153 EDTMP |
|
Average dose at 70 kg |
~4.0 mCi |
70 mCi |
Flare Reaction |
Yes - but % unknown |
Yes - ~ 7% |
Myelosuppression recovery |
10 - 16 weeks |
8 weeks |
Pain Relief |
65 - 80 % |
~ 50% |
Length of pain relief |
3 - 6 months |
As long as 11 months |
Imaging |
No |
Yes |
Re-injection |
Yes |
Yes |
Other Therapeutic Radiopharmaceuticals - Not FDA Approved
- Rhenium – 186 hydroxyethylene Disphosphonate (186Re HEDP)
- Properties
- Production: 185Re(no,γ)186Re
- Similar to 99mTc-HEDP/HDP in the compounding
- SnCl2 is the reducing agent and binding requires it to be placed in a 10 minute water bath at 100oC
- Beta particle maximum energy is 1.07 MeV
- Gamma ray energy is 137 keV with a 9% abundant
- Physical T1/2 = 90 hours
- Via chemabsorption HEDP replaces phosphate on the hydroxyapetite crystal
- An article by Liepe and Kotzerke compares 186Re/188ReHEDP to 89Sr and 153Sm. The conclusion showed no significant advantage of one agent over the other in its relief of bone pain
- Literature review
- Some form of pain relief occurs in approximately 77% of patients treated
- Dose range seems most effective at 50 to 65 mCi
- Application are for metastatic breast and prostate cancers
- Marrow production drops at 4-5 weeks and returns by 8 weeks post dose
- 188RheniumHEDP
- Generator
- 188W parent has a 69 day T1/2
- 188Re daughter has a 16.9 hour T1/2
- Properties
- Similar to 186ReHEDP
- Maximum beta energy = 2.2 Mev
- 155 keV gamma at 15% abundance
- Literature review
- Dose range 35 - 50mCi
- Myelosuppression occurs 50% of the time
- Response to treatment 63%
- 117mTin(Stannic, +4) Diethylenetriaminepentaacetic Acid (117mSn DTPA )
- Properties
- Production:117mSn(No,γ)117mSn
- Has a 14 day T1/2
- Decays by isomeric transition and the conversion electron emits 156 keV gamma (86% abundance)
- conversion electron causes low level ionization and does not use beta radiation
- Literature review
- Few studies have been done on this agent with data that can be dated to the late 1990s
- Patients receiving 131-156 :Ci/kg dose had the best response
- 77% of the dose remains in the bone 14 days after injection
- 85% of patient received at least some form of pain relieve
- No marrow toxicity was observed
- Pilot study completed by Krishnamurthy, et al. in 1997
- The above images shows the tin therapy agent (L) at one day post dose and the other whole body image is a 3 hour delay MDP scan. The patient has prostate cancer
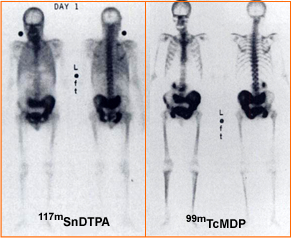
http://jnm.snmjournals.org/cgi/reprint/38/2/230 - It is a mystery as to why this agent has not been further investigated
Return to the beginning of the documents
Return to the Table of Contents