- The most commonly occurring hematological cancer in the US that ranks sixth in leading cancer death
- Approximately 98,982 people died of NHL between 1990 to 1994. In 2004, the estimate is around 21,000
- For more information on cancer mortality rates in US go to the following link: http://www.cdc.gov/cancer/index.htm (select the type of cancer and then statistics)
- Histologically there are three grades of NHL (low, medium, and high). This ranking is related to the progression of the disease, relapse after chemotherapy, and the patient's life expectancy
- Known as immunotherapy there are specific monoclonal antibodies that treat this type of cancer. Why does Bexxar work?
- It recognizes a specific protein found on a tumor cell
- Without a radioactive tracer this MoAb can binds to a specific tumor cell resulting in apoptosis(cell death)
- This MoAb targets the CD20 antigen located on the surface of malignant and normal lymphocytes
- Radioimmunotherapy application's use with beta radiation
- By targeting just the tumor cells it significantly reduces the radiation burden to the patient
- Once the MaAb is attached to the tumor 131I -beta particles travel approximately 0.8 mm in every direction, (~ 70 to 80 times the diameter of a lymphocyte). This results in a "crossfire effect" to the the surround tumor cells
- Because of specific targeting, healthly cells/tissue receive less radiation. End result reduces radiation burden to the patient

- The above diagram shows the development of B-lymphocytes
over time and helps us understand how/why this type of therapy works
- Under ideal situations, the MoAb is stable, tumor-specific, and attaches to the surface of the Antigen
- The key to attacking tumor cells is its CD20 expression
- Under normal developement of lymphocytes the undifferentiated stem cell does not have the CD20 expression, hence the radiation burden to those cells are not "effected." In addition, the final point on B-cell development (plasma cell) no longer has the CD 20 expression. HOwever, the Pre-B to Acitve B cell does have the CD20 expression
- Those cells that have the CD20 (normal and tumor) will undergo radioimmunotherapy when this agent is injected
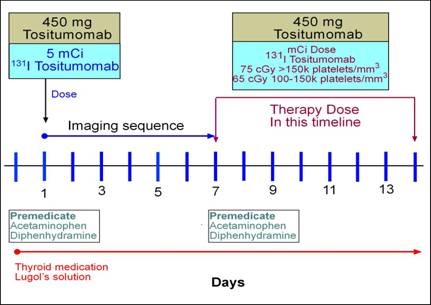
- The therapeutic dose
- Maximum total body dose should be between 65 - 75 cGy
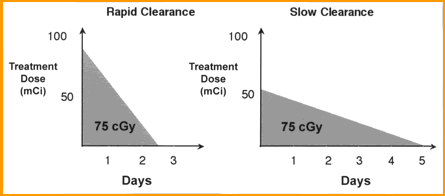
- At issue is that the biological T1/2 varies between patients
- Hence, it is important to determine how quickly a patient eliminate the radio-moab
- The faster the body eliminate the MoAb the higher the dose must be in order to receive the same therapeutic dose
- Consider the variation of 131I given to patients that has hyperthyroidism (size of the gland and uptake is taken into account). With Bexxar a similar principle is applied
- How is the therapeutic dose determined?
- Initially, 5mCi of 131I-tositumomab is given to the patient
- 131I standard is pulled off to the side and used to help calculate the patient's dose
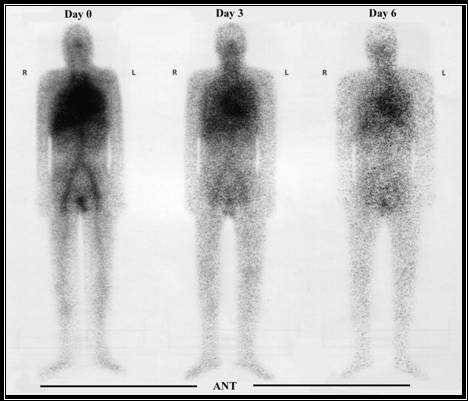
- On the 2nd, 3rd, or 4th day a whole-body scan is acquired and is usually collected within a 2-minute a time frame. The concern is not resolution, but count distribution
- To higher resolutions scans are completed on day 1 and 6 or 7
- All data must be acquired with a computer
- Patient counts, standard counts, and background counts are determined from both whole body scans
- Standard is used as QC, to take into account possible variations in the gamma camera's ability to pickup counts over the different times that the whole body scan is acquired (consider the use of a standard in thyroid uptake - its the same principle)
- Patient background counts are used to determine dose residence time
- Key - determine when the activity drops to 37% from the initial administration determines the dose given to the patient
- Above is an example on how this would be done. Note - Standard dose to calculate % efficiency is first applied, the 5 mCi dose is converted to dpm, multiplied by the efficiency and then multiplied by 37%.

(3)
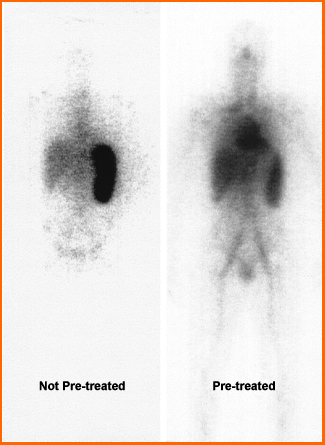
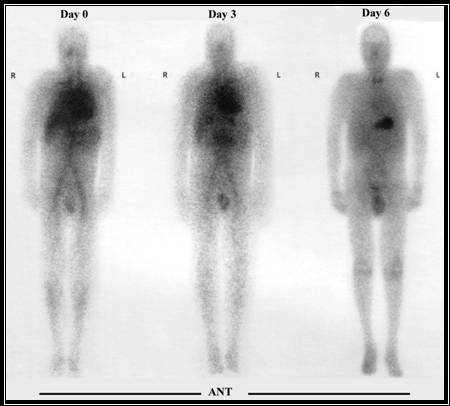
- Notice the difference between the pre-treatment (tositumomab) image and non pre-treated
- The non-treated image shows that a significant portion of the radiotracer has gone to then spleen
- If the same process was used in a therapeutic administration of tositumomab, too much of the radiotracer would radiate the wrong cells and tissues
- Therefore, pre-treatment will
- Reduces a immune cytotoxic response via cold moab binds to the non tumor B-cells, hence blocking uptake of the radioactive moab
- Questions - So if it blocks the healthily B-cells does it not also block the cancer cells that have the CD 20 expression?
- Multi center single-arm study
- Forty patients were initially studied
- Overall, 68% of the patients responded to the treatment
- 33% after the patients studied had no disease at 26 months post dose
- Collimator for 364 keV gamma with less than 7% septal penetration
- Single or dual detectors with whole body imaging capacity
- Use 20 - 25% window
- Use minimum whole body matrix if more than one is available
- Scan speed 10-30 cm/minute, however for pre dosing see above
- Prior to any radioactive dose potassium iodine must be administered
- Dosing
- OD - One day prior
- OD - Continue for fourteen days
- Dosing is specifically calculated for each patient and is determined by the initial 5mCi dose, see above. Once the dosimetry is determined the following platelet counts defines the actual dose.
- Platelets/mm 3 is >150k patient’s whole body dosimetry should be 75 cGy
- Platelets/mm 3 is between 150k-100k patient patient’s whole body dosimetry should be 65 cGy
- Platelets/mm 3 is <100k should not undergo treatment
- Calculation of this dose will be discussed in clinic