CHEM L110 - Chemistry and Society Laboratory
INTRODUCTION
Vitamins are required for good health. One of the essential vitamins is Vitamin C. A normal diet that includes fresh fruits, fruit juices, and vegetables typically supplies the necessary amount of vitamin C that an individual needs on a daily basis. However, vitamin C tablets are also widely used as nutritional supplements. Some people advocate taking large doses of vitamin C to combat the common cold.
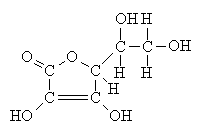
Vitamin C is ascorbic acid (C6H8O6). Its structure is shown below.

Scurvy plagued sailors from the time of Columbus through the 19th century. It was eventually recognized that a diet including citrus fruits prevented this disease. Today, vitamin C is viewed as an important water soluble "antioxidant". An antioxidant is a chemical compound that readily donates electrons. This property allows an antioxidant to block potentially harmful free radical reactions. To maintain good health, the Food and Drug Administration recommends a daily value of vitamin C of 60 milligrams (60 mg).
In this experiment, you will analyze, by titration, several samples of fruit juice to determine how much vitamin C each sample contains. You will also analyze a vitamin C tablet. The titration reaction for vitamin C (ascorbic acid) takes advantage of its ability to donate electrons to an appropriate acceptor. For this titration, iodine (I2) is the electron acceptor. The titration reaction is shown below:
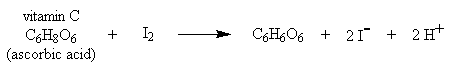
In this titration, starch is used as the end point indicator. The end point of the titration is signaled by the appearance of a blue or black color.
The amount of ascorbic acid (mg/mL) in a sample can be determined from the following equation:
![]()
EXPERIMENTAL PROCEDURE
Vitamin C Sample Titrations
You must use the same pipet for the vitamin C and iodine solutions for all of your titrations. The pipet must be rinsed with deionized H2O anytime you switch between any of these solutions.
You will be using a white ceramic wellplate for all the titrations in this experiment. Make sure your wellplate is clean and dry before you start.
There are six samples to titrate. Work in groups of two. One person will analyze a vitamin C tablet solution, Minute Maid Orange Juice, and Tropicana Orange Juice. The partner will analyze Sunny Delight, Hi C, and Kool-Aid. You are to copy your partner's data and do calculations for your own data as well as your partner's data.
1. Get four small, clean and dry test tubes. Label them to identify your vitamin C samples and the I2 solution. Fill each test tube approximately half-full with your three vitamin C samples and the I2 solution. Bring the samples back to your lab bench.
2. Using your pipet, add the indicated number of drops (see report sheet) of one particular vitamin C sample solution to five different wells in one row of your wellplate. Be careful not to contaminate the solution in one well with solutions from any other well.
3. Add two drops of starch indicator solution to each of the five wells.
4. Using a plastic pipet, add iodine solution slowly, one drop at a time to the vitamin C sample solution in the first well. Count the drops of iodine solution as you add them. Stir the solution after the addition of each drop. Continue adding iodine solution dropwise until, after stirring, the dark color remains. This signals the end of the titration. Record the number of drops of iodine solution used.
5. Do this titration at least five times. The number of drops of iodine solution used for each of the five titrations should agree with each other within three drops. If they do not, repeat the titration(s) until you have five results that agree with each other within this limit. Cross out the results that are too high or too low. Do not use them in your calculations.
6. Calculate the average number of drops of iodine solution used in the five titrations.
7. Calculate the average amount of vitamin C (in mg/mL) for this sample (using eqn 1).
8. Repeat steps 2 - 7 for your second sample (see report sheet).
9. Repeat steps 2 - 7 for your third sample (see report sheet).
10. Using the formulas on your report sheet, calculate
i) the mg of ascorbic acid per vitamin C tablet.
ii) the mg of ascorbic acid per 8 ounce serving of each of the five juices