CHEM L110 - Chemistry and Society Laboratory
INTRODUCTION
Acids and bases are two important classes of chemical compounds. Aqueous solutions of acids contain excess H+ ions, while aqueous solutions of bases contain excess OH- ions. It is important to be able to determine whether or not a substance is an acid or a base. In addition, we would like to be able to determine how acidic or how basic a substance is. There are a number of ways this can be done.
Litmus paper can qualitatively tell you whether a substance is acidic, basic, or neutral. pH is a quantitative measure of acid and base strength. The pH scale goes from 0 to 14. A solution with a pH of 7 is neutral. Solutions with pH less than 7 are acidic. Solutions with pH greater than 7 are basic. The lower the pH, the more acidic is the solution. The higher the pH, the more basic is the solution. pH can be measured in a number of different ways. For instance, you might use pH paper, Universal indicator, or an electronic pH meter.
In this experiment you will test rain along with a number of other common substances. You will first decide whether a substance is acidic, basic, or neutral. Litmus paper will then be used to determine whether the substance is acidic, basic, or neutral. Finally, you will use a natural pH indicator, the juice from a red cabbage, to determine its pH.
EXPERIMENTAL PROCEDURE
I. Use of Neutral Litmus Paper
Place a small piece of litmus paper on a paper towel on the lab bench. Using a plastic pipet, place one drop of the solution to be tested on the litmus paper. If the color of the litmus paper changes to some shade of pink, the solution is acidic. If the color of the litmus paper changes to some shade of blue, the solution is basic. If there is no change in color, the solution is neutral. Be careful not to confuse wet litmus paper alone as a color change.
II. Red Cabbage Juice pH Indicator
Fill your 100 mL beaker half full of deionized water. Add several pieces of red cabbage and bring the liquid to a boil. This will extract the natural dye from the red cabbage. The color of the red cabbage juice is different for different pH values. This allows it to be used as a pH indicator. You will use this solution to determine the pH of each substance in this experiment.
III. Wellplate Setup
You will be using a white ceramic wellplate for all the measurements in this experiment. Make sure your wellplate is clean and dry before you start.

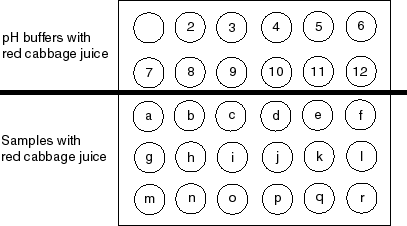
1. A buffer is a special solution with a specific pH. In this experiment there are 11 different buffer solutions with pH values from 2 through 12. Using a plastic pipet, add 15 drops of pH = 2 buffer into the well labeled "2". Using a different plastic pipet, add 15 drops of pH = 3 buffer into the well labeled "3". Continue this process until you have added a different buffer solution to each of the 11 labeled wells in the top two rows of the wellplate. The pH of the buffer solution and the well number (figure above) it is in must match.
2. Add 15 drops of red cabbage juice to each of the buffer solutions. The red cabbage juice will change color depending on the buffer pH.
IV. Sample Testing
See the table on the Report Sheet for the samples to be tested.
1. Before testing any sample, indicate whether you think the sample is acidic (A), basic (B), or neutral (N). On the report sheet place an A, B, or N in the first data column.
2. Use neutral litmus paper (see I above) to determine whether your sample is acidic (A), basic (B), or neutral (N). On the report sheet place an A, B, or N in the second data column.
3. Using a plastic pipet (except for samples c, h, and i), add 15 drops of the sample into the appropriate well. Match the sample letter with the well letter. For samples c, h, and i, add a drop or two to the well using the sample bottle itself.
4. Add 15 drops of red cabbage juice to each sample. Match the color of the sample solution to the color of one of the buffer solutions. The pH of the sample tested is the pH of the buffer solution it matches in color. Record the pH in the last data column.