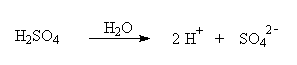
Rinse your 50 mL beaker with deionized water. Place 2 mL of H2SO4 solution into the beaker. Measure and record the conductivity.
CHEM L110 - Chemistry and Society Laboratory
INTRODUCTION
In the reaction between a metal and a nonmetal, the metal loses one or more electrons becoming a cation (Mn+) while the nonmetal gains one or more electrons becoming an anion (Xn-). The resulting compound is called an ionic compound. Some common examples of ionic compounds are NaCl (sodium chloride), CaCl2 (calcium chloride), and KBr (potassium bromide). When a soluble ionic compound, like NaCl, is dissolved in water, it breaks apart into Na+ and Cl- ions. Because these ions are free to move throughout the solution, and because the ions carry electric charges, the solution will conduct electricity. Electrical conductivity (conductance) provides a convenient experimental procedure to test for the presence of ions. In addition, the magnitude of the conductance is proportional to the concentration of ions in the solution.
In this experiment you are going to use a conductivity detector 1) to measure the conductivity of several liquids, solutions, and foods; 2) to observe the effect of ion concentration on conductivity; and 3) to measure conductivity changes during a chemical reaction.
EXPERIMENTAL PROCEDURE
Using the Conductivity Detector
The conductivity detector used in this experiment is adapted from D. A. Katz and C. Willis, J. Chem. Ed., 71, 330-2, 1994.
Paper clip wire will be used as electrodes. Attach a partially unfolded paper clip to each alligator clip on the
conductivity detector. The paper clip electrodes can then be inserted into the
solutions to be tested. To avoid corrosion, never dip the alligator clips themselves into
any solution.
To test a liquid sample, dip the electrodes
into the liquid (they must not touch each other in the solution) and observe the red and
green light-emitting diodes (LED). Use the table below to assign a conductivity value
(0 to 4) to your sample.
Conductivity Scale for Conductivity Measurements
| Red LED | Green LED | Conductivity | Scale |
| off | off | very low or none | 0 |
| dim | off | low | 1 |
| medium | off | medium | 2 |
| bright | dim | high | 3 |
| very bright | medium | very high | 4 |
The higher the conductivity, the higher the ion concentration in the solution. It is important to rinse off the electrodes with deionized water each time they are inserted into a new solution to prevent contamination.
I. Conductivity of Liquids and Solutions
Rinse your 50 mL beaker with deionized water. Place 4 mL of the appropriate sample into the beaker. (See the table in part I of the report sheet for the samples to be tested.) Measure and record the conductivity. Repeat this procedure for each sample.
II. Conductivity and Ion Concentration
Rinse your 50 mL beaker with deionized water. Attach partially unfolded paper clip electrodes to the inside of the beaker. Place 2 mL of deionized water into the beaker. To the water, add a NaCl solution 2 drops at a time. After every 2 drops gently swirl the beaker to mix the solution. Measure and record the conductivity after every 2 drops.
III. Conductivity Changes During a Chemical Reaction
IIIA. Sulfuric acid, H2SO4,
is called a strong acid. In aqueous solution, H2SO4 dissociates into
ions according to the following equation:
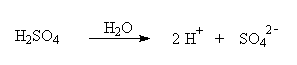
Rinse your 50 mL beaker with deionized
water. Place 2 mL of H2SO4 solution
into the beaker. Measure and record the conductivity.
Barium hydroxide, Ba(OH)2, is
called a strong base. In aqueous solution, Ba(OH)2 dissociates into ions
according to the following equation:

Rinse your 50 mL beaker with deionized
water. Place 2 mL of Ba(OH)2 solution into the
beaker. Measure and record the
conductivity. Save this solution for part IIIB.
IIIB. Ba(OH)2 and H2SO4
react with each other according to the following equation:
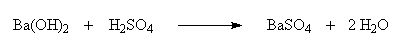
Another way to represent this reaction is:
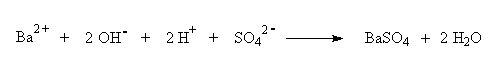
As a result of this reaction, ions (Ba2+, OH-, H+, and SO42-) are removed from the solution by producing the insoluble BaSO4 and H2O. Because BaSO4 is insoluble in water, it does not dissociate into ions. After all of the Ba(OH)2 has reacted, continued addition of H2SO4 puts ions (H+ and SO42-) back into solution.
Attach partially unfolded paper clip electrodes to the inside of the beaker containing the Ba(OH)2 solution from part IIIA. Add a H2SO4 solution 1 drop at a time. After each drop gently swirl the beaker to mix the solution. Measure and record the conductivity after each drop.
IV. Conductivity of Foods
See the table in part IV of the report sheet for the food samples to be tested. Insert the paper clip electrodes directly into the food sample. Keep the electrodes about the same distance apart for each food sample. Measure and record the conductivity. Rinse the electrodes with deionized water after testing each food sample.