CHEM L110 - Chemistry and Society Laboratory
INTRODUCTION
We live in an ocean of gases. Of the three states of matter (solids, liquids, and gases), gases are the most disordered. Gases have no shape or volume of their own. A gas adopts the shape and volume of its container. Gas molecules are far apart from each other and travel at high speeds. They collide with each other, with us, and with the walls of their container. This results in the pressure that a gas exerts.
The ocean of gases that we live in is our atmosphere. It contains 78% nitrogen (N2) and 21% oxygen (O2) with the remaining 1% consisting mainly of argon (Ar) and carbon dioxide (CO2). Photosynthetic bacteria, particularly cyanobacteria, are responsible for much of the oxygen found in the atmosphere. In photosynthesis, carbon dioxide and water are converted into carbohydrates and oxygen. The energy stored in carbohydrates and other hydrocarbons can be released by "burning" them in the presence of oxygen. In your body, these carbohydrates are "burned" in the presence of oxygen that you breathe. In addition to producing energy, this reaction also produces carbon dioxide and water. This carbon dioxide is found in your exhaled breath. Carbon dioxide is known as a greenhouse gas.
In this experiment you will prepare samples of oxygen and carbon dioxide gas. In addition you will examine the chemical properties of oxygen, carbon dioxide, exhaled air, and room air.
EXPERIMENTAL PROCEDURE
I. Preparation of Carbon Dioxide (CO2)
Carbon dioxide gas can be prepared by the reaction of sodium bicarbonate, NaHCO3, with hydrochloric acid, HCl.
![]()
1. Carefully measure out a teaspoonful of sodium bicarbonate, NaHCO3. Place this reactant in the corner of a small (1 pint) plastic "zipper" freezer bag.
2. Fill a plastic pipet as full as possible with 20% hydrochloric
acid, HCl.
(Caution: HCl is a corrosive acid.)
3. Without squeezing, place the pipet with the HCl into the plastic bag containing the NaHCO3. Orient the pipet so the narrow tip is pointing toward the NaHCO3. To remove as much air as possible from the bag, flatten the sides together. Seal the bag.
5. Carefully observe what happens. Touch the bag where the reaction is taking place. Record your observations.
6. The plastic bag contains carbon dioxide (CO2) gas. Keep it sealed when you are not taking out CO2 samples.
II. Preparation of Oxygen (O2)
Oxygen gas can be prepared by the catalytic decomposition of hydrogen peroxide, H2O2. The catalyst used here is yeast.
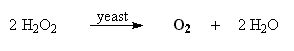
1. Using a graduated cylinder, measure out 25 mL of 3% hydrogen peroxide, H2O2.
2. Pour the H2O2 solution into an empty small (1 pint) plastic "zipper" freezer bag.
4. Carefully observe what happens. Touch the bag where the reaction is taking place. Record your observations.
5. The plastic bag contains oxygen (O2) gas. Keep it sealed when you are not taking out O2 samples.
III. Exhaled Air
1. Take a deep breath and then exhale into an empty small (1 pint) plastic "zipper" freezer bag. Repeat if necessary. Seal the bag.
2. The plastic bag contains exhaled air. Keep it sealed when you are not taking out exhaled air samples.
IV. Testing the Properties of Carbon Dioxide, Oxygen, Exhaled Air, and Room Air
IVa. Reaction with Limewater
This test is to determine the relative amount of carbon dioxide (CO2) in a gas sample. Limewater is an aqueous solution of calcium hydroxide, Ca(OH)2. It reacts with carbon dioxide according to the equation:
![]()
You will look for the formation of a white solid which is calcium carbonate (CaCO3). The amount of white solid (CaCO3) that appears in the solution is proportional to the amount of CO2 in the gas sample.
Do this test on all four gases.
1. Put 4 small clean and dry test tubes in a test tube rack. Place 10 to 15 drops of limewater (Ca(OH)2) solution in each test tube.
2. Use a new clean plastic pipet for each gas sample.
3. To fill a pipet with a sample of a particular gas, squeeze the pipet bulb to expel the air inside. While still squeezing the bulb, have your lab partner make a small opening in the "zipper" seal of the plastic bag. Insert the pipet tip into the bag, but do not touch the liquid or solid with the pipet. With the pipet in the bag, release the bulb to draw gas into the pipet. Remove the pipet and quickly re-seal the bag.
4. Take the pipet filled with carbon dioxide and place it in the first test tube. With the pipet tip below the surface of the solution, slowly squeeze the pipet bulb to bubble the gas through the limewater solution. Record your observations.
5. Get a sample of oxygen gas in a clean pipet. Bubble it through the limewater in the second test tube. Record your observations.
6. Get a sample of exhaled air in a clean pipet. Bubble it through the limewater in the third test tube. Record your observations.
7. To get a sample of room air, use a clean pipet. Squeeze and release the pipet bulb several times to draw in room air. Bubble it through the limewater in the fourth test tube. Record your observations.
IVb. Reaction with Phenol Red Indicator
This is a qualitative test to determine whether a particular gas sample reacts with water to produce an acidic solution. The faster the indicator changes color from light red to light yellow, the more acidic is the solution.
Do this test only on carbon dioxide and exhaled air.
1. Phenol red is an acid/base indicator. Place ~30 drops of phenol red indicator solution in a small clean and dry test tube. Keep the test tube lightly corked between samplings.
2. The reaction vessel for this test will be the pipet bulb itself. Squeeze and hold the bulb of a new clean plastic pipet. Place the pipet into the phenol red solution in the test tube. Slowly release the bulb and allow the phenol red solution to fill only the barrel of the pipet. Remove the pipet from the solution. Turn the pipet upside down to allow the solution to run into the bulb. If any solution remains in the barrel, gently squeeze the bulb to expel it (do this over a sink).
3. To sample carbon dioxide gas, hold the pipet with the tip pointing up and squeeze the bulb to expel the air but not the phenol red solution. While still squeezing the bulb, have your lab partner make a small opening in the "zipper" seal of the plastic bag. Insert the pipet tip into the bag, but do not touch the liquid or solid with the pipet. With the pipet in the bag, release the bulb to draw gas into the pipet. Remove the pipet and quickly re-seal the bag.
Gently shake the pipet to mix the carbon dioxide gas with the phenol red indicator solution. Record your observations.
4. Repeat steps 2 & 3 using a sample of exhaled air. Record your observations.
IVc. Flammability of Carbon Dioxide and Oxygen (Demonstration).
Carbon Dioxide. Prepare a new sample of carbon dioxide gas (see Procedure I). Light the end of a wooden splint. Let it burn for a few seconds. Have your partner open the plastic zipper bag filled with carbon dioxide. Quickly insert the burning splint. Record your observations.
Oxygen. Prepare a new sample of oxygen gas (see Procedure II). Light the end of a wooden splint. Let it burn for a few seconds. Blow it out so that the end is just glowing. Have your partner open the plastic zipper bag filled with oxygen. Quickly insert the glowing splint. Record your observations.