Acarbose
|
Sections |
| Structure |
| Mechanism of Action |
| Questionnaire |
Acarbose is one of the new class of oral a-glucosidase inhibitors. It is an antidiabetic and is useful for Type II patients who do not respond to diet alone.
Acarbose is a linear tetrasaccharide of a-D-glucose residues modified at the non-reducing end. The non-reducing end group saccharide residue has a nitrogen replacing the interglycosidic oxygen atom and a double bond between C-4 and C-5 positions. The structure is as follows and to view its 3D images click here or on the structure.
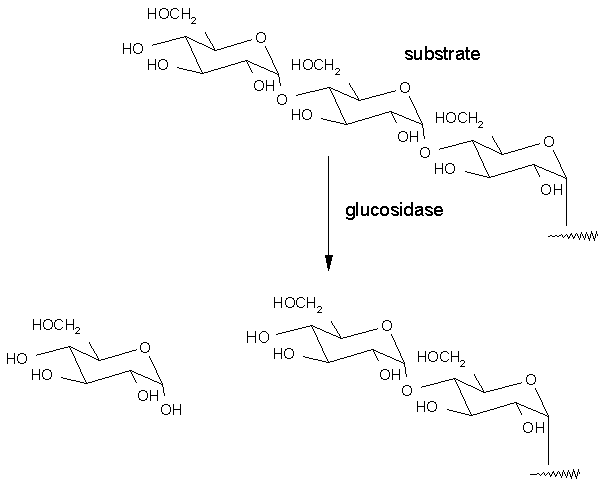
In a typical reaction in the intestine the polymeric sugar chain is broken down into individual monosaccharide units by the action of a-glucosidase. (see below) This enzyme recognizes specific residues in the polymeric sugar chain and hydrolyses the interglycoside linkage through the formation of an oxonium ion intermediate as shown below. Subsequent hydrolyses of the intermediate releases the non-reducing end residue (monosaccharide) which becomes available for quick absorption in the intestine and release in the blood.

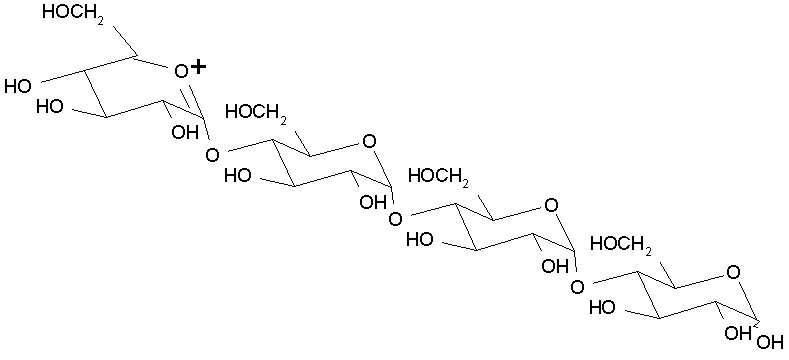
Acarbose is a stable transition state inhibitor of this reaction (see below). Its structure resembles the transition state structure of the polysaccharide and is devoid of its ability to form a stable oxonium ion intermediate. Acarbose has a 105-fold higher affinity than typical substrates (Ki ~10-12 M). Thus, acarbose binds the a-glucosidase enzyme incapacitating it from its action on its normal substrates. This prevents the release of monosaccharides from polysaccharides in the stomach and intestine. The polysaccharide is rather eliminated in feces.
[session home] [home] [school
of pharmacy] [department of medicinal
chemistry]
©2000 VCU School of Pharmacy
Revised: January 15, 2000
Questions or Comments : Dr. Umesh
R. Desai