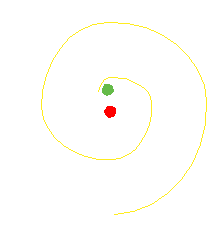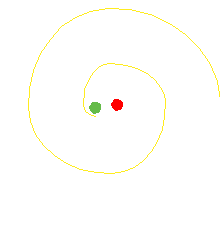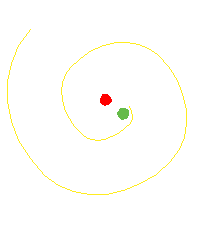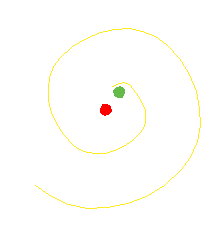
 The orbiting electron in the Bohr atom should radiate electromagnetic waves until the electron spirals in to the nucleus.
The orbiting electron in the Bohr atom should radiate electromagnetic waves until the electron spirals in to the nucleus. The orbiting electron in the Bohr atom should radiate electromagnetic waves until the electron spirals in to the nucleus.
The orbiting electron in the Bohr atom should radiate electromagnetic waves until the electron spirals in to the nucleus.
It does not do that.
![]()
The radiation should have a frequency related to the orbital period but the Bohr model states instead that the frequency is related to the energies of the allowed orbits by Planck's formula: