- Some radiopharmacies set their 99mTc-chelate to 95% or greater percent bound before allowing it for patient administration
- What are the other components found in solution after a 99mTc radiopharmaceutical is compounded?
- 99mTc7+ is reduced by Sn2+, usually to 99mTc4+
- There can still be some free 99mTc7+ in solution, 99mTcO4-
- Hydrolyzed 99mTc can occur in solution, to a by-product 99mTcO2. This usually occurs when there is an excessive amount o Sn2+. It can form 99mTc Sn(OH)2 stannous hydroxide. (This is a colloid)
- Once 99mTcO2 is formed it cannot chelate to the intended radiopharmaceutical
- Stannous ion can also become a colloid in the presence of moisture
- In the presences of air (O2) reduced technetium can re-oxidize and revert back to 99mTcO4-
- Some kits have ascorbic acid (antioxidant) to help retard the re-oxidation process
- More information about Sn can be found by clicking
- Radionuclide Purity – This is the fraction of the total desired radiopharmaceutical after compounding. Can you think of different examples?
- Radiochemical purity – Is the total amount or faction of the desired radioactive compound. Can you think of different examples?
- What about 99Mo in 99mTc, how would this effect our imaging results if too much Molly is in solution?
- Could radiolysis be an issue? What would happen if this occurred?
- Stability of the tag can be effected by time, light, temperature, or radiolysis
- Closer look at the ITLC strips
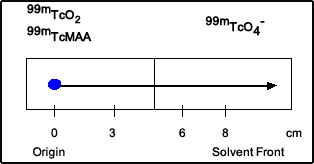
- A drop of the radiopharmaceutical is placed at the origin on the strip indicated by the blue dot
- The strip is then placed in a tube that contains a specific type of solvent
- The type of solvent varies depends on the type of radiopharmaceutical used
- The goal is to use a solvent that can separate the different compounds found in the radiopharmaceutical solution
- After the strip is placed in the solvent and via capillary action, the solution moves up the strip, indicated by the arrow
- As the solvent moves the different compounds in the radiopharmaceutical separate based on how the a solvent effects the agent
- Strip is then taken out after the solvent travels to the top
- The point of were the drop is placed is known as the origin
- The end point is known as the solvent front
- Reference front (Rf) value is then identified by finding the position on of a specific compound and determining its location on the strip
- There are two methods used to determine this the Rf value
- Usually you can cut the ITLC strip in half and determine the amount of activity
- Or if the department has a radiochromatographic scanner activity is counted along the strip and then the radioactive distribution can be graphically displayed
- Below is an image of a radiochromatographic scanner
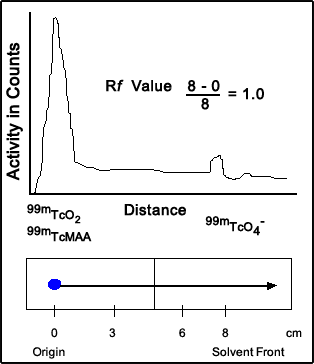
- From the strip above 99mTc-MAA was placed at the origin. The solvent used was acetone. HR 99mTc and 99mTc-MAA stay at the origin while 99mTcO4- moves to the top of the solvent front
- Rf values for each component is noted (assume the distance between the solvent front and the origin is 10 cm)
- 0 for HR 99mTc (stayed at the origin 0cm/10cm = 0) - this one is not a concern since MAA does not dissolve in aqueous solution
- 0 for 99mTcMAA (stayed at the origin 0cm /10cm = 0)
- 1.0 for 99mTcO4- (moved to the end of the solvent front 10cm/10cm = 1.0)
- Percent of bound can now be calculated by determining the amount of activity at Rf = 0 and at Rf = 1.0
- See diagram below
- Other points of interest
- Instant Thin-Layer Chromatography = ITCL are glass fiber impregnated with
- Silica gel ITLC (SG) or
- Polysilic acid ITLC (SA)
- Stationary phase are those components that do not move along the solvent front
- Mobile phase are those components that move along the solvent front
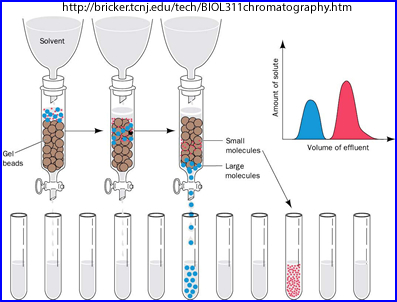
- Gel chromatography
- A sample or drop is placed at the top of a column containing Sephadex gel which is soaked in a specific solvent (based on what needs to be separated)
- Separation of the radiopharmaceutical is dependent on molecular size, where the larger molecules move faster through the gelled solution
- Each fraction of the solution is then measured and % bound is determined
- An example of separation could be: 99mTc-MDP come out first(tube A), then 99mTc04- second (tube B), and 99mTc02 (R - remains)remains in the column. So how would you calculate %Bound? Assume A has 57,900 cpm, B has 1577 cpm, R has 995 cpm. Answer
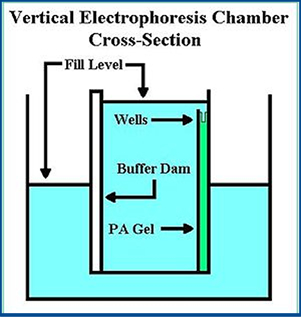
- Paper or polyacrylamide gel electrophoresis
- Small sample of radiopharmaceutical is applied to the gel or paper
- Soaked in a buffer
- Voltage is applied across this material for a period of time
- Based on charge and ionic mobility the components within the radiopharmaceutical separate and percentages can be calculated
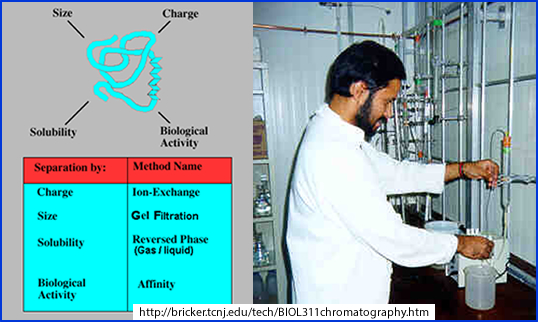
- Ion Exchange
- Sample of radiopharmaceutical is placed on in a column of ionic resin and the column is eluted with an appropriate solvent
- Different species are separated by the exchange of ions from the solution and the resin
- Percentages can then be calculated
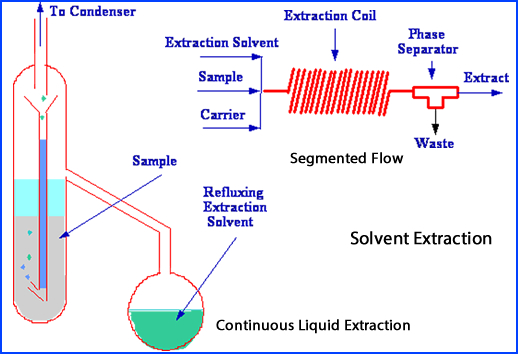
- Solvent extraction
- Radiopharmaceutical is placed into an liquid and shaken
- Separation of the radiopharmaceutical within the solution depends on preferential solubility of the compounds or elements that are in solution
- The different components within the radiopharmaceutical separate into different immiscilbe liquids
- These components can assessed for the amount of radiochemical purity
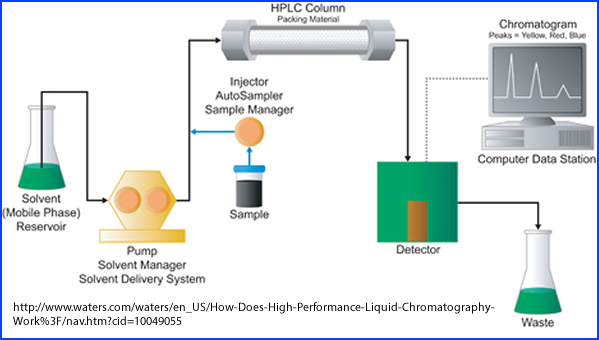
- High-performance liquid chromatography (HPLC)
- Radiopharmaceutical is placed in a column containing packing material and solution
- The sample is then forced through a column via electrical pumps
- Different solutions are passed though this system at high pressure where separation of the radioactive components occur
- This results is a high resolution speed separation as the different components within each solution are assayed
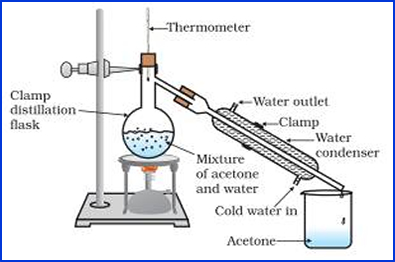
- Distillation
- Via high vapor pressure radiopharmaceutical is distilled leaving other compounds
- Best for determining free iodine or noble gases
http://www.ncerthelp.com/text.php?contype=Concept&class_id=9&sub_id=S&chapter_id=CH2&q_no=13
- This is defined as the fraction of material desired
- Al+3 in a 99mTcO4- is an example
- Additive in solution such as reducing and anti-oxidizing agents are not considered impurities
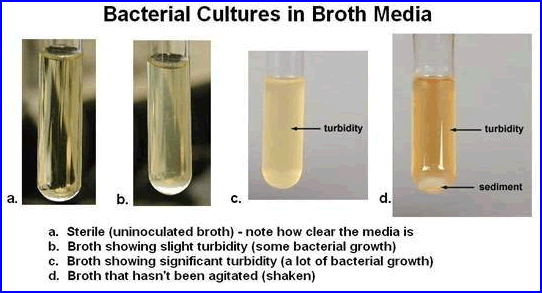
- Sterility
- There is/are no viable bacteria or microorganisms present
- Several methods are employed to assure sterility
- Autoclaving
- Water is heated to 121oC creating steam that is pressurized at 18 psi for 15 minutes
- Destroys microorganisms
- Certain radiopharmaceuticals cannot tolerate this method since it will break down the compound (ex. Protein). Hence a radiopharmaceutical must be thermostable
- This technique is not useful with short lived radionuclides (ex. PET)
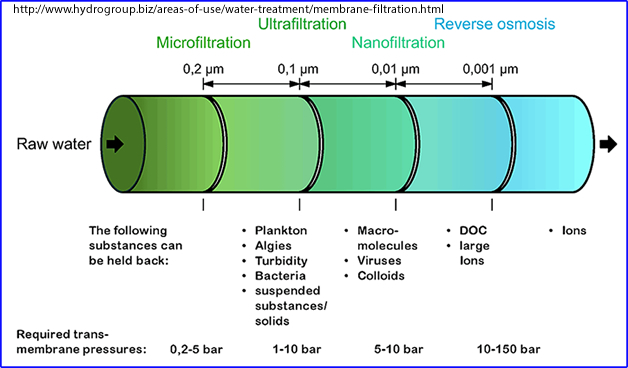
- Membrane filtration
- Filtering a radiopharmaceutical to remove microorganisms
- Milli-pore filter most commonly used is 0.45 μm, however, 0.22 μm may also be used to filter out smaller components (ex. Blood)
- Method is best used for short-lived radiopharmaceuticals
- Method is also used to generate filtered colloid - Do you recall the filter's size?
- Sterility testing
- Method I - Take a radiopharmaceutical sample and place into a thioglycollate media. Incubate at 30 – 35oC for 14 days
- Method II - Use a soybean-casein digest media and incubate at 20 – 25oC for 14 days
- These methods are usually done by the manufacturer after the pharmaceutical has been made
- For shorter lived radionuclides 14C-glucose in a trypticase soy broth culture. Generation of 14C-glucose in a gas that ionization chamber indicates that presence of microorganisms. This process only takes 3 -24 hours
- Pyrogens or endotoxins
- While a radiopharmaceutical may be sterile after or before compounding there can be residual components left from bacteria:
- Pyrogens cause fever or reaction that may be associated with the endotoxin and are fragments of bacterial cell wall. Reactions may occur: fever, chills, malaise, leukopenia, pain in the joints, flushing, sweating, headache, and/or pupil dilation. Usually they are composed of polypeptides
- Endotoxins is a subclass of Pyrogens and are reminiscence of gram-negative bacteria cell wall, specifically lipopolysaccharides, may be considered poisonous, and will cause fever
- Symptoms occur between 30 minutes and 2 hours post dose and usually dissipate 10 to 12 hours later
- Pyrogenicity testing
- USP Rabbit Test
- Three rabbits are kept in a controlled environment and injected with a radiopharmaceutical
- Dose is calculated based on weight, 3 – 10 times the human equivalent
- Rectal temperatures are taken at 1, 2, and 3 hours post injection
- Less than 0.6oC change per rabbit or nothing greater than 1.4oC summed means there are no endotoxins present
- If positive, then the test is repeated with 5 rabbits where less than 0.6oC change per rabbit or a sum of less than 3.7oC indicates the lack of endotoxins
- LAL test (Limulus amebocyte lysate)
- Limulus polyphemus is extracted from the blood of a Horseshoe Crab
- Usually 0.l mL of LAL is mixed with the radiopharmaceutical and incubated at 37oC for 15 -60 minutes at a pH between 6 to 8
- If the compound turns opaque gel it is considered positive. The thicker the gel the greater the amount of pathogens
- Toxicity
- Does the radiopharmaceutical have a toxin effect on the human system?
- Example of this would be thallium is toxic to the human body, so how can we use it?
- Animals are injected (mice, rat, rabbit, or dog) with the radiopharmaceutical. After 2 to 6 weeks the animals are scarified and an autopsy is done to evaluate the animal’s systems.
- LD 50/30 - NRC defines the median death rate in humans
- Two types of animals are given radioactive doses at increasing levels
- This establishes when 50% die at 30 days
- However, this test has, for the most part, been replaced with cell cultures and computer modeling
- Here are some other facts from WIKI

https://www.quora.com/What-is-turbidity-in-regards-to-microbiology

