- Alpha particle:α
- Beta particle (positron and negatron): β + and β -
- Gamma ray/x-ray: γ-ray and χ-ray
- Disintegrations are measures in time
- Usually expressed in disintegrations per second or minute (dps or dpm).
- These disintegrations are are in the form gamma radiation
- α, β +, and β - may also be released from an atom
- Tri-linear chart shows radioactive decay in a different way
- β- moves downward because there are too many neutrons
- β+ moves upward because there are too many protons
- When it reaches the center the atoms are no longer radioactive. This is where the hexagon is black
- Chart gives other data as well: T1/2, energy levels, decay schemes
Review of the particles
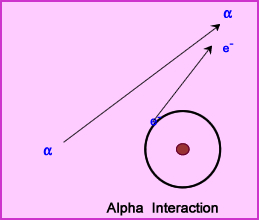
- Alpha particles are ejected from the nucleus of an atom
- Contains 2 - protons and 2 - neutrons
- Considered very damaging to life
- When alpha particles are ejected from the nucleus, two neutrons and two protons are released (a) and given a certain amount of kinetic energy
- Kinetic energy released from the ejected alpha particle allows it to passes through the media causing ionization events by attracting electrons
- Alpha particles travel a short distance with nothing greater than 4 cm in air
- Inhaled, swallowed, or with direct contact can cause significant damage to the surface of an organ/system
- At rest mass α becomes a Helium atom, by attracting two electrons
- Shielding for alpha can be done with just a piece of paper? Why?
- The good news is that in general nuclear medicine does not use this type of radiation. However, there is one exception to this rule, Xofigo, which therapeutically treats metastatic prostate cancer

- Neutron rich nuclei decay because there are too many neutrons
- This radioactive decay process converts a a neutron to proton and releases a beta minus particle
- Considered a negatively charged electron
- N ---->P + β- + anti-v (neutrino)
- Anti-neutrino is also ejected and carries part of the energy from the decay reaction
- Beta particle and the anti-neutrino share this kinetic energy, which is the kinetic energy that allows it to be ejected from the nucleus
- β- ionizes the media by repelling outer shell shell electron. This causes the beta particle to loose energy and ionizes the atom by ejecting the electron from its outer orbit [see above diagram]
- At rest mass the β- particle becomes an electron
- ß- particles can be shielded with either wood and plastic
- Lead may also be used to shield when additional gamma radiation being emitted from the nucleus (referred to as a mix emitter)
- If Pb shielding is on a pure beta emitter bremsstrahlung radiation will occur
- Bremsstrahlung radiation (in German it means breaking radiation)

- This occurs when accelerated particles encounter material that have a high Z number (density). Example:
- Accelerated particle is a beta
- Example of a high Z number material would be lead
- When a beta particle encounters lead the highly packed molecules cause the beta particle to slow down quickly (interaction between the electrons in the lead and the positively or negativity charged beta particle)
- This causes x-ray emissions
- Should beta decaying radionuclides be stored in lead or plastic?
- Consider a radionuclide that has both beta and gamma decay – what is the best way to shield this type of radioactive material?
- Proton rich nuclei decay by converting protons to neutrons
- Proton rich atoms decay as follows - P+ ----> No + β+ + ν (neutrino)
- Decay in this manner causes converted of a proton to neutron and the release of β+(positron) and a neutrino (ν). If there is any energy left over then gamma ray(s) may also be released
- initial energy imparted to the β+ ejects it from the nucleus
- As it passes an outer shell electron, it causes one or more ionizations events (just like alpha, but not as intense)
- It will continue to ionize the surrounding media until all its energy is gone. When no kinetic energy remains, it then comes to rest mass
- At rest mass the positive electron meets up with an electron and an annihilation event occurs

- When the β+ reaches rest mass it attracts an e-
- This event converts mass to energy and releases two 511 keV (photons moving in opposite directions (180o)
- Shielding for β+can be achieved with either plastic or wood
- Lead(or other high density material) must be used to shield the 511 keV photons
- Occurs when there is too much energy in the nucleus, it is considered to be in an excited state
- The release of gamma ray energy is usually the end process from another decay
- Relate this to beta minus/plus decay .. usually there is additional energy left in the nucleus. This additional energy is then released from the nucleus in the form of gamma radiation
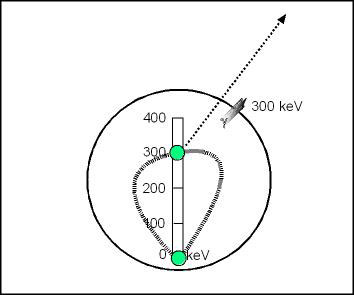
- Assume there is still 300 keV of energy remaining in the nucleus after a beta decay
- The nucleus raised to this higher energy state cannot be maintained.
- This results in energy being released from the nucleus, in the form of a γ-ray, that would have 300 keV of energy
- The nucleus the return to its ground stated, making it more stable
- This is what happens in a isomeric reactions where the nucleus is meta-stable (99mTc ------>99Tc)
- Electrons within the obit of an atom receive excess energy when an electron is knocked out
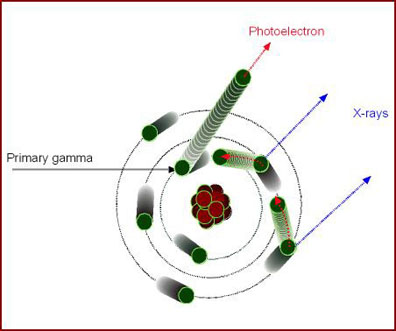
- As the higher shell of electrons move in to fill in the the missing electron, x-rays are released. This is what happens in Photoelectric Effect where χ-rays are produced
- What is the difference/similarities between a gamma and an x-ray?
- Gamma rays originate from the nucleus of an atom
- X-rays originate from an electron shell
- Neither have weight or charge-like properties
- Its properties allow x and gamma rays to travel a lot farther than alpha and beta particles
- Distanced travel depends on the amount of kVp/keV and the surrounding attenuation
- Interact with matter occurs when it runs into something (usually an electron and at energies >1.024 meV the nucleus)
- Lead is required to stop these rays
- At rest mass nothing is left
Gamma Ray Interaction With Matter

- A higher energy gamma will interact with the media via a process known as the Compton Effect
- Primary gamma interacts with an outer shell electron
- This creates a secondary gamma and an ejected electron
- This is referred to as a Compton event/electron
- The greater degree of scatter (max = 180 degrees) the greater the energy imparted to the Compton electron and the less keV energy given to the secondary gamma - think cue ball's interaction in a pool
- As the gamma energy reduces (via Compton) its energy it eventually interact in a processes known as Photoelectric Effect
- Primary gamma interacts with an inner shell electron
- All the energy is given to the electron creating a photoelectron
- Higher orbiting electrons then move into lower obits creating χ-rays
- Thompson or characteristic radiation occurs at lower level energy where the kinetic energy of the gamma is lower than the binding energy of an electron
- Gamma ray will interact with the electron
- Electron is not ejected
- Secondary gamma ray is released, with no loss of energy