
|
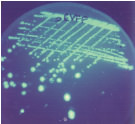 Your trick is simple. You fuse the gene encoding UDP glucose dehydrogenase to that encoding green fluorescent protein (GFP). If the enzyme interacts to produce a precipitate, then GFP will get pulled into the precipitate as well and fail to fluoresce. A mutant enzyme that fails to precipitate will leave the tethered GFP free as well, giving rise to a highly fluorescent colony.
Your trick is simple. You fuse the gene encoding UDP glucose dehydrogenase to that encoding green fluorescent protein (GFP). If the enzyme interacts to produce a precipitate, then GFP will get pulled into the precipitate as well and fail to fluoresce. A mutant enzyme that fails to precipitate will leave the tethered GFP free as well, giving rise to a highly fluorescent colony.
That's the theory. In fact what you get are a few rare colonies that show moderately more fluorescence than the unmutated fusion. These rare colonies show less precipitate, but there's still a lot. You're going in the right direction, but you clearly need to go further -- further than random mutagenesis can take you. Photo courtesy of Clare Chemical Research |